NHS Information Authority
Data Standards Programme
| Reference: |
Change Request 315 |
| Version No: | 1.9 |
| Subject: | DG1126 - Review of KC65 - Colposcopy |
| Type of Change: | Change to NHS data standards |
| Effective Date: | 1 April 2003 |
| Reason for Change: | Data Standards to support revisions to the KC65 return. |
Background:
DSCN 04/2003 brought in revisions to the KC65 central return for Colposcopy Clinic referrals, treatments and outcomes, effective from 1 April 2003. This DSCN makes the necessary changes to NHS data standards to support the revised KC65 as follows: For Part A of the return, the classification of COLPOSCOPY REFERRAL INDICATION has been updated to reflect the change to Clinical Indication.
For Part B, ATTENDED OR DID NOT ATTEND has been extended, and a new attribute APPOINTMENT TYPE introduced.
The description of COLPOSCOPY PRIME PROCEDURE TYPE has been modified to reflect Parts C1 and C2; and PATIENT INFORMED BIOPSY RESULT DATE has been changed to reflect Part D.
Minor changes have been made to BIOPSY REFERRAL OUTCOME and COLPOSCOPY PRIME PROCEDURE TYPE for Part E.
Changes have also been made to support the move from exclusively consultant activity, and to include nurse clinics.
Summary of changes:
| Name: | Kevin Shine |
| Date: | 11 March 2003 |
| Sponsor: | Data Standards Team |
Note: Additions shown in highlighted with a blue background. Deletions are shown using strikeout.
CLINIC ATTENDANCE NURSE
Change to Class:
change to attributes
Attributes of this Class are:
top
OUT-PATIENT APPOINTMENT
Change to Class:
change to attributes
Attributes of this Class are:
top
APPOINTMENT TYPE
Change to Attribute:
New Attribute
APPOINTMENT TYPE
The type of an OUT-PATIENT APPOINTMENT for a colposcopy clinic. Note that there may be more than one appointment of type 'first appointment'.
Classification:
|
| a. | First appointment :
An appointment for a first attendance |
| b. | Treatment:
A follow-up appointment specifically for treatment |
| c. | Follow-up/surveillance:
All other follow-up appointments |
This attribute is also known by these names: | Context | Alias |
|---|
| plural | APPOINTMENT TYPES |
top
ATTENDED OR DID NOT ATTEND
Change to Attribute:
change to description
This indicates whether or not a PERSON or PATIENT attended for an appointment. If the PATIENT did not attend it also indicates whether or not advanced warning was given.
For colposcopy appointments only, code 2 is subdivided into:
(i) before the appointment date
(ii) on the appointment day
and code 3 is subdivided into:
(i) arrived, but did not wait to be seen
(ii) other
National Codes:
| 5 | Attended on time or, if late, before the relevant health care professional was ready to see the patient |
| 6 | Arrived late, after the relevant health care professional was ready to see the patient, but was seen |
| 7 | Patient arrived late and could not be seen |
| 2 | Appointment cancelled by, or on behalf of, the patient |
| 3 | Did not attend - no advance warning given |
| 4 | Appointment cancelled or postponed by the HEALTH CARE PROVIDER |
Note: The classification has been listed in logical sequence rather than alphanumeric order.
This attribute is also known by these names: | Context | Alias |
|---|
| plural | ATTENDED OR DID NOT ATTEND |
top
BIOPSY REFERRAL OUTCOME
Change to Attribute:
change to description
A code used to reference an outcome of a REFERRAL FOR BIOPSY. For cervical histology, biopsies are taken after a colposcopy has been performed.
For cervical histology for KC61 purposes, the classification is:
| a. | Cervical Cancer (including micro-invasive) |
b. | Adenocarcinoma in situ |
| b. | Adenocarcinoma in situ / CGIN |
| c. | CIN3 |
| d. | CIN2 |
| e. | CIN1 |
| f. | HPV only |
| g. | No CIN/No HPV |
| h. | Inadequate biopsy |
| i. | Colposcopy - Nothing Abnormal Detected (NAD)/no biopsy taken |
| j. | Results not known by laboratory |
| k. | Non cervical cancers detected |
For cervical histology for KC65 purposes, the classification is:
| a. | Cancer (including micro-invasive) |
| b. | Adenocarcinoma in situ |
| c. | CIN3 |
| d. | CIN2 |
| e. | CIN1 |
| f. | HPV/cervicitus only |
| g. | No CIN/No HPV (normal) |
| h. | Inadequate/unsatisfactory biopsy |
| i. | Result not known by clinic |
For breast cancer screening for KC62 purposes,the classification is:
| a. | Benign or negative |
| b. | Inconclusive |
| c. | Positive; i.e. cancer detected |
| | i. | non-invasice or possibly micro-invasive |
| | | a. | low (DCIS only detected) |
| | | b. | intermediate (DCIS only detected) |
| | | c. | high (DCIS only detected) |
| | | d. | grade not known (DCIS only detected) |
| | ii. | definitely micro-invasive |
| | | a. | low (DCIS only detected) |
| | | b. | intermediate (DCIS only detected) |
| | | c. | high (DCIS only detected) |
| | | d. | grade not known (DCIS only detected) |
| | iii. | invasive size not known |
| | | a. | Grade I |
| | | b. | Grade II |
| | | c. | Grade III |
| | | d. | grade not known |
| | iv. | invasive size known |
| | | a. | Grade I |
| | | b. | Grade II |
| | | c. | Grade III |
| | | d. | grade not known |
| | | v. | invasive status not known |
d. | Biopsy not done or result not yet know |
References:
KC65 - Colposcopy Clinics, Referrals, Treatments and Outcomes
KC61: Pathology Laboratories - Cervical Cytology and Outcome of Gynaecological Referrals
KC62: Adult Screening Programmes - Breast Screening
This attribute is also known by these names: | Context | Alias |
|---|
| plural | BIOPSY REFERRAL OUTCOMES |
top
COLPOSCOPY PRIME PROCEDURE TYPE
Change to Attribute:
change to description
The COLPOSCOPY PRIME PROCEDURE TYPES identifies the prime PATIENT PROCEDURE undertaken during the first Colposcopy Clinic OUT-PATIENT ATTENDANCE CONSULTANT. The COLPOSCOPY PRIME PROCEDURE TYPE identifies the prime PATIENT PROCEDURE undertaken during the first Colposcopy CLINIC ATTENDANCE CONSULTANT or CLINIC ATTENDANCE NURSE within the REPORTING PERIOD. More than one procedure may be carried out, but only one should be recorded for KC65 purposes, this being determined by the order of severity. The classifications are listed in order of severity with the most severe being listed first and so on.
Note where the PATIENT opts to defer treatment to a later time the classification e. No treatment; no treatment received and no biopsy taken, should be recorded.
Classification:
a. | Ablation; treatment method recorded as ablation and biopsy type recorded as other than no biopsy. This will include cold coagulation, cryotherapy, cautery and diathermy. |
| a. | Ablation; treatment method recorded as ablation. This will include cold coagulation, cryotherapy, cautery and diathermy. |
| (i) biopsy result available |
| (ii) no biopsy taken, or biopsy result not known by clinic |
| b. | Loop/laser excision or knife cone; treatment method recorded as loop/laser excision or knife cone and biopsy type recorded as other than no biopsy. This will include LLETZ and NEEP. |
c. | Diagnostic biopsy (punch); no treatment received and biopsy type recorded as directed biopsy or multiple directed biopsy. |
| c. | Diagnostic biopsy (punch); no treatment received and biopsy type recorded as directed biopsy or multiple directed biopsy, or any other biopsy taken for diagnostic purposes only. |
| d. | Other; treatment method recorded as other and biopsy type recorded as other than no biopsy. This will include polyp avulsion and treatment with silver nitrate. |
| e. | No treatment; no treatment received and no biopsy taken. |
This attribute is also known by these names: | Context | Alias |
|---|
| plural | COLPOSCOPY PRIME PROCEDURE TYPES |
top
COLPOSCOPY REFERRAL INDICATION
Change to Attribute:
change to description
The referral indication of a REFERRAL REQUEST to a Colposcopy Clinic.
Classification:
| a. | Screening smear |
| | i. | Abnormal screening smear |
| | ii. | Abnormal smear after colposcopy |
| b. | Clinical indication |
| | i. | Clinically suspicious cervix |
| | i. | Urgent |
| | ii. | Suspicious symptoms |
| | ii. | Non-urgent |
This attribute is also known by these names: | Context | Alias |
|---|
| plural | COLPOSCOPY REFERRAL INDICATIONS |
top
PATIENT INFORMED BIOPSY RESULT DATE
Change to Attribute:
change to description
The date the patient was informed of the result of a biopsy taken as a result of a colposcopy PATIENT PROCEDURE. The date the patient was informed in writing of the result of a biopsy taken as a result of a colposcopy PATIENT PROCEDURE.
This attribute is also known by these names: | Context | Alias |
|---|
| plural | PATIENT INFORMED BIOPSY RESULT DATES |
top
KC65 1
Change to Central Return Form:
Change guidance text
| Central Return Form Guidance |
KC65 - Colposcopy Clinics: Referrals, Treatments and Outcomes
Contextual Overview
- The Department, NHS Cervical Screening Programme (NHSCSP) and Regional Offices require information from NHS HEALTH CARE PROVIDERS on colposcopy clinic activity.
The Department, NHS Cervical Screening Programme (NHSCSP) and Regional Offices require information from NHS HEALTH CARE PROVIDERS on colposcopy clinic activity.
The KC65 will form part of the wider NHS Cancer Information Strategy which aims to improve the effectiveness and efficiency of care delivery for those with actual or suspected cancer, throughout the patient journey.
- The KC65 forms part of the wider NHS Cancer Information Strategy which aims to improve the effectiveness and efficiency of care delivery for those with actual or suspected cancer, throughout the patient journey.
The information is used to monitor the process of achieving the Government's target to reduce the incidence of invasive cervical cancer and to monitor the performance of colposcopy clinics on local, regional and national levels.
- The information is used to monitor the process of achieving the Government's target to reduce the incidence of invasive cervical cancer and to monitor the performance of colposcopy clinics on local, regional and national levels.
Information on the return is also used in Public Expenditure Survey (PES) negotiations, resource allocation to the NHS and Departmental accountability.
- Information on the return is also used in Public Expenditure Survey (PES) negotiations, resource allocation to the NHS and Departmental accountability.
Information based on the KC65 return is published annually by the Department in the Statistical Bulletin Cervical Screening Programme.
- Information based on the KC65 return is published annually by the Department in the Statistical Bulletin Cervical Screening Programme.
Completing Return KC65 - Colposcopy Clinics: Referrals, Treatments and Outcomes
KC65 is a quarterly return with the first quarter starting on 1 April and the last quarter ending on 31 March. Returns must be submitted by the thirtieth working day after the end of the quarter.
- KC65 is a quarterly return with the first quarter starting on 1 April and the last quarter ending on 31 March. Returns must be submitted by the thirtieth working day after the end of the quarter.
The KC65 return requires the ORGANISATION CODE and ORGANISATION NAME of the NHS HEALTH CARE PROVIDER - NHS Trust or Primary Care Trust - as well as the name of a contact and the contact telephone number on the front page. It must be signed and dated by the person completing the return.
- The KC65 return requires the ORGANISATION CODE and ORGANISATION NAME of the NHS HEALTH CARE PROVIDER - NHS Trust or Primary Care Trust - as well as the name of a contact and the contact telephone number on the front page. It must be signed and dated by the person completing the return.
The British Society for Colposcopy and Cervical Pathology has agreed a Minimum Data Set (MDS) for colposcopy services, currently being introduced into Colposcopy Clinics. The MDS meets professional requirements for audit and quality improvement as well as departmental needs, and provides the information needed to complete the KC65.
- The British Society for Colposcopy and Cervical Pathology has agreed a Minimum Data Set (MDS) for colposcopy services, currently being introduced into Colposcopy Clinics. The MDS meets professional requirements for audit and quality improvement as well as departmental needs, and provides the information needed to complete the KC65.
Colposcopy
Colposcopy is a PATIENT PROCEDURE carried out on a woman who has been referred to a Colposcopy Clinic following a SCREENING TEST carried out either as part of a SCREENING PROGRAMME or opportunistically. Alternatively the woman may be referred as a result of clinical indications.
Colposcopy is a PATIENT PROCEDURE carried out on a woman who has been referred to a Colposcopy Clinic following a SCREENING TEST carried out either as part of a SCREENING PROGRAMME or opportunistically. Alternatively the woman may be referred as a result of clinical indications.
top
KC65 2
Change to Central Return Form:
Change guidance text
| Central Return Form Guidance |
KC65 - Colposcopy Clinics: Referrals, Treatments and Outcomes
Part A - Women referred to colposcopy by referral indication and result of referral
Part A - Women referred to colposcopy by result of referral smear and time from referral to first appointment
Part A of the KC65 return is a count of the number of women referred for colposcopy. This information is used to monitor referral patterns to ensure that guidelines on referral are being followed.
- Part A of the KC65 return is a count of the number of women referred for colposcopy. This information is used to monitor referral patterns to ensure that guidelines on referral are being followed.
A colposcopy is a PATIENT PROCEDURE carried out during a CLINIC ATTENDANCE CONSULTANT on a PATIENT. The PATIENT will have been referred to the Colposcopy Clinic:
In cases where there is both a clinical indication and a SCREENING TEST referral smear, the referral should be treated as clinical indication.
The data is based on the woman's first OUT-PATIENT APPOINTMENT CONSULTANT in the quarter regardless of whether she attended the clinic or not.
Please note that the total number referred as recorded in Part A should equal the total number waiting as recorded in Part B as both parts relate to the same cohort of women.
Referral Indication - Screening smear (line 0001)
- A colposcopy is a PATIENT PROCEDURE carried out during a CLINIC ATTENDANCE CONSULTANT or CLINIC ATTENDANCE NURSE. The PATIENT will have been referred to the Colposcopy Clinic:
In cases where there is both a clinical indication and a SCREENING TEST referral smear, the referral should be treated as clinical indication.
- The data is based on the woman's first OUT-PATIENT APPOINTMENT in the quarter regardless of whether she attended the clinic or not.
Time from referral to first appointment (lines 0001 to 0005)
This line counts all the women with a REFERRAL REQUEST for coloposcopy with a COLPOSCOPY REFERRAL INDICATION of Screening smear. These are PERSONS IN A SCREENING PROGRAMME who have been given a SCREENING TEST as part of a planned SCREENING PROGRAMME. It also includes women screened opportunistically, these women have had a SCREENING TEST with the OPPORTUNISTIC SCREENING INDICATOR of Yes.
In addition, if a PERSON IN A SCREENING PROGRAMME has been suspended from the SCREENING PROGRAMME following colposcopy and is currently having surveillance smears as indicated by SCREENED WHILE SUSPENDED INDICATOR of Yes, it may be that an abnormal smear will cause the woman to be re-referred to colposcopy. In this case the COLPOSCOPY REFERRAL INDICATION should be Screening smear, regardless of whether or not she has been discharged from colposcopy at this time.
Referral Indication - Clinical indication (line 0002)
- Lines 0001 to 0005 are counts of REFERRAL REQUESTS by the time from referral to first appointment. This should be measured from the PATHOLOGY RESULT REPORTED DATE for referrals following a screening test, and from the REFERRAL DATE for all other REFERRAL REQUESTS, to the APPOINTMENT DATE of the first OUT-PATIENT APPOINTMENT.
For patients with an appointment of APPOINTMENT TYPE First appointment which was cancelled by the clinic (ATTENDED OR DID NOT ATTEND was Appointment cancelled or postponed by the HEALTH CARE PROVIDER), the time is measured from referral to the subsequent First appointment.
Referral Indication - Result of referral smear (columns 2 to 8)
This line counts all the women with a REFERRAL REQUEST for coloposcopy with a COLPOSCOPY REFERRAL INDICATION of Clinical indication.
Where a woman is referred with symptoms and is given a SCREENING TEST the COLPOSCOPY REFERRAL INDICATION should still be Clinical indication and not Screening smear. Where no symptoms are present the COLPOSCOPY REFERRAL INDICATION should not be Clinical indication.
Results of referral smear
The information in columns 2-9 is based on the cervical screening test results, which led to the REFERRAL REQUEST. Classifications are those of CYTOLOGY RESULT TYPES of a REQUEST FOR PATHOLOGY INVESTIGATION and are in accordance with the categories shown in box 22 of HMR 101/5 Request/Report for Cervical or Vaginal Cytology.
Entries recorded in Other (column 9) should only occur in exceptional circumstances. NHS Cervical Screening Programme (NHSCSP) guidelines state that all smears should be identified as belonging to one of the eight recognised category classifications of CYTOLOGY RESULT TYPE. Other (column 9) does not correspond to these recognised categories and should be used to record those rare cases in which a recognised category is not appropriate. Where an entry is present in Other (column 9) then supporting notes should be recorded in the available box on the first page of the KC65 form.
Where the cervical screening test results which led to the REFERRAL REQUEST indicates more than one result type, the most severe result should recorded as the CYTOLOGY RESULT TYPE.
- These columns count all the women with a REFERRAL REQUEST for colposcopy with a COLPOSCOPY REFERRAL INDICATION of Screening smear. These are PERSONS IN A SCREENING PROGRAMME who have been given a SCREENING TEST as part of a planned SCREENING PROGRAMME. It also includes women screened opportunistically, these women have had a SCREENING TEST with the OPPORTUNISTIC SCREENING INDICATOR of Yes.
In addition, if a PERSON IN A SCREENING PROGRAMME has been suspended from the SCREENING PROGRAMME following colposcopy and is currently having surveillance smears as indicated by SCREENED WHILE SUSPENDED INDICATOR of Yes, it may be that an abnormal smear will cause the woman to be re-referred to colposcopy. In this case the COLPOSCOPY REFERRAL INDICATION should be Screening smear, regardless of whether or not she has been discharged from colposcopy at this time.
- The information in columns 2-8 is based on the cervical screening test results, which led to the REFERRAL REQUEST. Classifications are those of CYTOLOGY RESULT TYPES of a REQUEST FOR PATHOLOGY INVESTIGATION and are in accordance with the categories shown in box 22 of HMR 101/5 Request/Report for Cervical or Vaginal Cytology.
Where the cervical screening test results which led to the REFERRAL REQUEST indicates more than one result type, the most severe result should recorded as the CYTOLOGY RESULT TYPE.
Inadequate (column 2)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of Inadequate sample (cat.1).
NHS Cervical Screening Programme guidelines state the recording of three cervical screening tests with a CYTOLOGY RESULT TYPEclassification of Inadequate sample (cat.1) indicates referral to colposcopy however, referral to colposcopy may occur following an inadequate smear for other reasons.
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Inadequate sample (cat.1).
NHS Cervical Screening Programme guidelines state the recording of three cervical screening tests with a CYTOLOGY RESULT TYPE classification of Inadequate sample (cat.1) indicates referral to colposcopy however, referral to colposcopy may occur following an inadequate smear for other reasons.
Borderline changes (column 3)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of Borderline changes (cat.8).
NHS Cervical Screening Programme guidelines state the recording of three cervical screening tests with a CYTOLOGY RESULT TYPEclassification of Borderline changes (cat.8) indicates referral to colposcopy however, referral to colposcopy may occur following a borderline smear for other reasons.
Mild dyskaryosis (column 4)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of Mild dyskaryosis (cat.3).
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Borderline changes (cat.8).
NHS Cervical Screening Programme guidelines state the recording of three cervical screening tests with a CYTOLOGY RESULT TYPEclassification of Borderline changes (cat.8) indicates referral to colposcopy however, referral to colposcopy may occur following a borderline smear for other reasons.
Mild dyskaryosis (column 4)
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Mild dyskaryosis (cat.3).
Moderate dyskaryosis (column 5)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of Moderate dyskaryosis (cat. 7), including abnormal, unclassifiable and ungraded smears.
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Moderate dyskaryosis (cat. 7), including abnormal, unclassifiable and ungraded smears.
Severe dyskaryosis (column 6)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of Severe dyskaryosis (cat.4).
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Severe dyskaryosis (cat.4).
Severe dyskaryosis/invasive carcinoma (column 7)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of Severe dyskaryosis/?invasive carcinoma (cat.5).
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Severe dyskaryosis/?invasive carcinoma (cat.5).
Glandular neoplasia (column 8)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of ?Glandular neoplasia (cat.6), including adenocarcinoma.
Other (column 9)
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of ?Glandular neoplasia (cat.6), including adenocarcinoma.
Referral Indication - Clinical indication (columns 9, 10)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of Other.
This should only be used in the rare situations where usual categorisation is not appropriate. Examples include women with incomplete or missing records and women who have moved from abroad.
Where an entry is present in Other (column 9) then supporting notes should be recorded in the available box on the first page of the KC65 form.
No referral smear (column 10)
- These columns count women with a REFERRAL REQUEST for colposcopy with a COLPOSCOPY REFERRAL INDICATION of Clinical indication.
Where a woman is referred with symptoms and is given a SCREENING TEST the COLPOSCOPY REFERRAL INDICATION should still be Clinical indication and not Screening smear. Where no symptoms are present the COLPOSCOPY REFERRAL INDICATION should not be Clinical indication.
Clinical Indication Urgent (column 9)
A count of the number of women who have been referred to the colposcopy clinic with a REFERRAL REQUEST with a COLPOSCOPY REFERRAL INDICATION of Clinical indication.
Total number referred (column 11)
- A count of the number of women with a COLPOSCOPY REFERRAL INDICATION of urgent. This is restricted to cervical lesions suspicious of cancer, or post-coital bleeding of over four weeks where the patient is aged over 35.
Clinical Indication Non-Urgent (column 10)
This is the total of women referred for colposcopy, split between with those referred with a COLPOSCOPY REFERRAL INDICATION of Clinical indication and those referred with a COLPOSCOPY REFERRAL INDICATION of Screening smear.
Total (line 0003)
- A count of the number of women with a COLPOSCOPY REFERRAL INDICATION of non-urgent. This includes all other symptomatic referrals for colposcopy.
Other (column 11)
This is the total for all women counted in columns 2 to 11.
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Other or with no CYTOLOGY RESULT TYPE and no COLPOSCOPY REFERRAL INDICATION.
Entries for a CYTOLOGY RESULT TYPE classification of Other should only occur in exceptional circumstances. NHS Cervical Screening Programme (NHSCSP) guidelines state that all smears should be identified as belonging to one of the eight recognised category classifications of CYTOLOGY RESULT TYPE. Other does not correspond to these recognised categories and should be used to record those rare cases in which a recognised category is not appropriate.
Otherwise this column should only be used in the rare situations where usual categorisation is not appropriate. Examples include women with incomplete or missing records and women who have moved from abroad.
Where an entry is present in column 11 supporting notes should be recorded in the available box on the first page of the KC65 form.
Total number referred (column 12)
- This is the total of women referred for colposcopy, broken down by time from referral to first appointment
Total (line 0006)
- This is the total for all women counted in columns 2 to 12.
top
KC65 3
Change to Central Return Form:
Change guidance text
| Central Return Form Guidance |
KC65 - Colposcopy Clinics: Referrals, Treatments and Outcomes
Part B - Women referred to colposcopy by result of referral smear and time from referral to first appointment
Part B - Appointments for Colposcopy
Part B of KC65 shows, for each referral, the waiting time before the first offered appointment. The NHS Cervical Screening Programme (NHSCSP) has issued guidance on waiting times, and the information is used to monitor whether clinics are meeting these standards.
- Part B of the KC65 return is a breakdown of appointments by cancellation/non-attendance, and type of appointment. This information will allow monitoring of non-attendances, patient cancellations, and clinic cancellations.
It includes all OUT-PATIENT APPOINTMENTS with an APPOINTMENT DATE within the REPORTING PERIOD.
Attendance Status
The waiting time is the length of time between the date on the referral letter - the REFERRAL DATE of the REFERRAL REQUEST for colposcopy and the woman's first OUT-PATIENT APPOINTMENT CONSULTANT in the quarter, regardless of whether she attended the colposcopy clinic or not. Where there has been a direct referral from the cytology laboratory, the referral date is the date the smear was reported - the SCREENING RESULT SENT DATE.
Please note that the total number referred as recorded in Part A should equal the total number waiting as recorded in Part B as both parts relate to the same cohort of women.
Time from referral to first appointment
Less than or equal to 2 weeks (line 0001)
- The Attendance status is derived from the value of ATTENDED OR DID NOT ATTEND for the OUT-PATIENT APPOINTMENT.
Attended (line 0001)
This counts the number of women whose waiting time was less than or equal to 14 days.
>2 weeks up to 4 weeks (line 0002)
- The number of appointments for which ATTENDED OR DID NOT ATTEND was either attended on time or, if late, before the relevant health care professional was ready to see the patient, or arrived late, after the relevant health care professional was ready to see the patient, but was seen.
Cancelled by patient - in advance (line 0002)
This counts the number of women whose waiting time was more than 14 days but less than or equal to 28 days.
>4 weeks up to 8 weeks (line 0003)
- The number of appointments for which ATTENDED OR DID NOT ATTEND was appointment cancelled by, or on behalf of, the patient - before the appointment date.
Cancelled by patient - on the day (line 0003)
- The number of appointments for which ATTENDED OR DID NOT ATTEND was appointment cancelled by, or on behalf of, the patient - on the appointment day.
Cancelled by Clinic (line 0004)
This counts the number of women whose waiting time was more than 28 days but less than or equal to 56 days.
>8 weeks up to 12 weeks (line 0004)
- The number of appointments for which ATTENDED OR DID NOT ATTEND was appointment cancelled or postponed by the HEALTH CARE PROVIDER.
DNA - no advance warning (line 0005)
This counts the number of women whose waiting time was more than 56 days but less than or equal to 84 days.
> 12 weeks (line 0005)
- The number of appointments for which ATTENDED OR DID NOT ATTEND was did not attend - no advance warning given (other).
DNA - arrived late (line 0006)
This counts the number of women whose waiting time was more than 84 days.
Results of referral smear
- The number of appointments for which ATTENDED OR DID NOT ATTEND was patient arrived late and could not be seen.
DNA - left without being seen (line 0007)
The information in columns 2-6 is based on the cervical screening test result which led to the REFERRAL REQUEST and directly relates to the information recorded in columns 2-9 of Part A of this form. Classifications are those of CYTOLOGY RESULT TYPES of a REQUEST FOR PATHOLOGY INVESTIGATION and are in accordance with the categories shown in box 22 of HMR 101/5 Request/Report for Cervical or Vaginal Cytology.
For Part B certain categories have been added together e.g. column 4 of Part B directly relates to column 5 and 6 of Part A.
Inadequate (column 2)
- The number of appointments for which ATTENDED OR DID NOT ATTEND was did not attend - no advance warning given (arrived, but did not wait to be seen).
Total (line 0008)
A count of the number of women with a CYTOLOGY RESULT TYPESclassification of Inadequate sample (cat. 1).
Borderline changes/Mild dyskaryosis (column 3)
- This is the total of all women counted in lines 0001 to 0007.
Appointment Type
A count of the number of women with a CYTOLOGY RESULT TYPESclassification of Borderline changes (cat. 8) or Mild dyskaryosis (cat. 3).
Moderate dyskaryosis/Severe dyskaryosis (column 4)
- Columns 2 to 4 require counts of colposcopy OUT-PATIENT APPOINTMENTS by APPOINTMENT TYPE.
New (column 2)
A count of the number of women with a CYTOLOGY RESULT TYPESclassification of Moderate dyskaryosis (cat. 7), including abnormal, unclassifiable and ungraded smears or Severe dyskaryosis (cat. 4).
Severe dyskaryosis/?invasive carcinoma/?Glandular neoplasia (column 5)
- The number of colposcopy OUT-PATIENT APPOINTMENTS where the APPOINTMENT TYPE is first appointment.
Return for Treatment (column 3)
A count of the number of women with a CYTOLOGY RESULT TYPESclassification of Severe dyskaryosis/?invasive carcinoma (cat. 5) or ?Glandular neoplasia (cat. 6), including adenocarcinoma.
Other (column 6)
- The number of colposcopy OUT-PATIENT APPOINTMENTS where the APPOINTMENT TYPE is treatment.
Follow Up (column 4)
A count of the number of women with a CYTOLOGY RESULT TYPESclassification of Other.
Clinical Indication (column 7)
- The number of colposcopy OUT-PATIENT APPOINTMENTS where the APPOINTMENT TYPE is follow-up/surveillance.
Total (column 5)
A count of the number of women who have been referred to the colposcopy clinic with a REFERRAL REQUEST with a COLPOSCOPY REFERRAL INDICATION of Clinical indication.
Total number referred (column 8)
This is the total of all women by length of time waiting.
Total (line 0006)
This is the total for all women counted in columns 2 to 8.
Data Quality Checks
The following data quality checks should be made:
Part A | Part B |
| | |
Line 003 Totals | = Line 006 Totals |
Column 2 Line 003 | = Column 2 Line 006 |
Column 3 + Column 4 Line 003 | = Column 3 Line 006 |
Column 5 + Column 6 Line 003 | = Column 4 Line 006 |
Column 7 + Column 8 Line 003 | = Column 5 Line 006 |
Column 9 Line 003 | = Column 6 Line 006 |
Column 10 Line 003 | = Column 7 Line 006 |
- This is the total for all women in columns 3 to 5.
top
KC65 4
Change to Central Return Form:
Change guidance text
| Central Return Form Guidance |
KC65 - Colposcopy Clinics: Referrals, Treatments and Outcomes
Part C - First attendances by type of procedure and result of referral
Part C1 - First attendances by type of procedure and result of referral
Part C of the KC65 return is a count of procedures undertaken at coloposcopy clinics, showing the nature of the initial treatment by result of referral. The information is used to monitor treatment patterns at first attendance to ensure that treatment guidelines, such as on the number of biopsies taken, are met.
- Parts C1 and C2 of the KC65 return are counts of procedures undertaken at colposcopy clinics, showing the nature of treatment by result of referral. The information is used to monitor treatment patterns to ensure that treatment guidelines, such as on the number of biopsies taken, are met.
Data is collected on the woman's first OUT-PATIENT APPOINTMENT CONSULTANT in the quarter (with FIRST ATTENDANCE classified as First Attendance).
Where a woman has a smear taken during the attendance the COLPOSCOPY PRIME PROCEDURE TYPE should be recorded as No treatment; no treatment received and no biopsy taken.
- Parts C1 and C2 are identical, except that Part C1 relates to initial treatment at first attendance, and Part C2 relates to all attendances. For part C1 data is collected on the woman's first CLINIC ATTENDANCE CONSULTANT or CLINIC ATTENDANCE NURSE in the REPORTING PERIOD (with FIRST ATTENDANCE classified as First Attendance).
Where a woman has a smear taken during the attendance the COLPOSCOPY PRIME PROCEDURE TYPE should be recorded as No treatment; no treatment received and no biopsy taken.
The procedures undertaken in the coloposcopy clinics are PATIENT PROCEDURES.Only one PATIENT PROCEDURE should be counted for each woman's FIRST ATTENDANCE. If more than one procedure is carried out, the most severe should be recorded for KC65.
- The procedures undertaken in the colposcopy clinics are PATIENT PROCEDURES. Only one PATIENT PROCEDURE should be counted for each woman's FIRST ATTENDANCE. If more than one procedure is carried out, the most severe should be recorded for KC65.
Result of referral smear
Lines 0001 to 0008 require data on the number of women referred for colposcopy by CYTOLOGY RESULT TYPES.
- Lines 0001 to 0008 require data on the number of women referred for colposcopy by CYTOLOGY RESULT TYPES.
Inadequate (line 0001)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of Inadequate sample (cat. 1).
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Inadequate sample (cat. 1).
Borderline changes (line 0002)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of Borderline changes (cat. 8).
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Borderline changes (cat. 8).
Mild dyskaryosis (line 0003)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of Mild dyskaryosis (cat. 3).
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Mild dyskaryosis (cat. 3).
Moderate dyskaryosis (line 0004)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of Moderate dyskaryosis (cat. 7), including abnormal, unclassifiable and ungraded smears..
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Moderate dyskaryosis (cat. 7), including abnormal, unclassifiable and ungraded smears..
Severe dyskaryosis (line 0005)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of Severe dyskaryosis (cat. 4).
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Severe dyskaryosis (cat. 4).
Severe dyskaryosis/invasive carcinoma (line 0006)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of Severe dyskaryosis/invasive carcinoma (cat. 5).
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Severe dyskaryosis/invasive carcinoma (cat. 5).
Glandular neoplasia (line 0007)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of ?Glandular neoplasia (cat. 6), including adenocarcinoma..
Other (line 0008)
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of ?Glandular neoplasia (cat. 6), including adenocarcinoma..
Referral Indication - Clinical indication (lines 0008, 0009)
A count of the number of women with a CYTOLOGY RESULT TYPEclassification of Other.
Entries recorded in Other (line 0008) should only occur in exceptional circumstances. NHS Cervical Screening Programme (NHSCSP) guidelines state that all smears should be identified as belonging to one of the eight recognised category classifications of CYTOLOGY RESULT TYPE. Other (line 0008) does not correspond to these recognised categories and should be used to record those rare cases in which a recognised category is not appropriate. Where an entry is present in Other (line 0008) then supporting notes should be recorded in the available box on the first page of the KC65 form.
Clinical Indication no referral smear (line 0009)
- These columns count first attendances for women with a REFERRAL REQUEST for colposcopy with a COLPOSCOPY REFERRAL INDICATION of Clinical indication.
Note all procedures carried out on women who have been referred to the colposcopy clinic with a REFERRAL REQUEST with a COLPOSCOPY REFERRAL INDICATION of Clinical indication should be recorded in this line regardless of the result of any smear taken after the referral
Clinical Indication Urgent (line 0008)
A count of the number of women who have been referred to the colposcopy clinic with a REFERRAL REQUEST with a COLPOSCOPY REFERRAL INDICATION of Clinical indication.
Note all procedures carried out on women who have been referred to the colposcopy clinic with a REFERRAL REQUEST with a COLPOSCOPY REFERRAL INDICATION of Clinical indication should be recorded in this line regardless of the result of any smear taken after the referral.
Total (line 0010)
- A count of the number of women with a COLPOSCOPY REFERRAL INDICATION of urgent. This is restricted to cervical lesions suspicious of cancer, or post-coital bleeding of over four weeks where the patient is aged over 35.
Clinical Indication Non-Urgent (line 0009)
This is the total for all women counted in columns 2 to 7.
- A count of the number of women with a COLPOSCOPY REFERRAL INDICATION of non-urgent. This includes all other symptomatic referrals for colposcopy
Other (line 0010)
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Other.
Entries recorded in Other (line 0010) should only occur in exceptional circumstances. NHS Cervical Screening Programme (NHSCSP) guidelines state that all smears should be identified as belonging to one of the eight recognised category classifications of CYTOLOGY RESULT TYPE. Other (line 0010) does not correspond to these recognised categories and should be used to record those rare cases in which a recognised category is not appropriate. Where an entry is present in Other (line 0010) then supporting notes should be recorded in the available box on the first page of the KC65 form.
Total (line 0011)
- This is the total for all women counted in columns 2 to 8.
No treatment (column 2)
This counts the number of women who received no treatment and for whom was recorded a COLPOSCOPY PRIME PROCEDURE TYPE of No treatment; no treatment received and no biopsy taken.
- This counts the number of women who received no treatment and for whom was recorded a COLPOSCOPY PRIME PROCEDURE TYPE of No treatment; no treatment received and no biopsy taken.
Procedure Type
Diagnostic biopsy (punch) (column 3)
This counts the number of women who received no treatment and for whom a COLPOSCOPY PRIME PROCEDURE TYPE of Diagnostic biopsy (punch); no treatment received and biopsy type recorded as directed biopsy or multiple directed biopsy was recorded.
Treatment biopsy or treatment/diagnostic biopsy - Ablation (column 4)
- This counts the number of women who received no treatment and for whom a COLPOSCOPY PRIME PROCEDURE TYPE of Diagnostic biopsy (punch); no treatment received and biopsy type recorded as directed biopsy or multiple directed biopsy, or any other biopsy taken for diagnostic purposes only was recorded.
Treatment biopsy or treatment/diagnostic biopsy - Excision (column 4)
This counts the number of women who for whom was recorded a COLPOSCOPY PRIME PROCEDURE TYPE of Ablation; treatment method recorded as ablation and biopsy type recorded as other than no biopsy. This will include cold coagulation, cryotherapy, cautery and diathermy.
Treatment biopsy or treatment/diagnostic biopsy - Excision (column 5)
This counts the number of women who for whom was recorded a COLPOSCOPY PRIME PROCEDURE TYPE of Loop/laser excision or knife cone; treatment method recorded as loop/laser excision or knife cone and biopsy type recorded as other than no biopsy. This will include LLETZ and NEEP.
Other (column 6)
- This counts the number of women who for whom was recorded a COLPOSCOPY PRIME PROCEDURE TYPE of Loop/laser excision or knife cone; treatment method recorded as loop/laser excision or knife cone and biopsy type recorded as other than no biopsy. This will include LLETZ and NEEP.
Ablation + No Biopsy taken or biopsy result not yet known (column 5)
This counts the number of women who for whom was recorded a COLPOSCOPY PRIME PROCEDURE TYPE of Other; treatment method recorded as other and biopsy type recorded as other than no biopsy. This will include polyp avulsion and treatment with silver nitrate.
Number of first attendances (column 7)
- This counts the number of women who for whom was recorded a COLPOSCOPY PRIME PROCEDURE TYPE of Ablation; treatment method recorded as ablation. This will include cold coagulation, cryotherapy, cautery and diathermy. (ii) no biopsy taken, or biopsy result not known by clinic.
Ablation + Biopsy (column 6)
This is the total of all first attendances (see paragraph 2), subdivided by the CYTOLOGY RESULT TYPEclassifications, except that the classification Other is included in with Clinical Indication No referral smear.
- This counts the number of women who for whom was recorded a COLPOSCOPY PRIME PROCEDURE TYPE of Ablation; treatment method recorded as ablation. This will include cold coagulation, cryotherapy, cautery and diathermy. (i) biopsy result available.
Other (column 7)
- This counts the number of women who for whom was recorded a COLPOSCOPY PRIME PROCEDURE TYPE of Other; treatment method recorded as other and biopsy type recorded as other than no biopsy. This will include polyp avulsion and treatment with silver nitrate. It excludes any treatment that is not related to cervical abnormalities.
Number of first attendances (column 8)
- This is the total of all first attendances (see paragraph 2), subdivided by the CYTOLOGY RESULT TYPE classifications.
top
KC65 5
Change to Central Return Form:
Change guidance text
| Central Return Form Guidance |
KC65 - Colposcopy Clinics: Referrals, Treatments and Outcomes
KC65 - Colposcopy Clinics: Referrals, Treatments and Outcomes
Part D - Biopsies, by time from biopsy to informing patient of result
Part C2 - All attendances by type of procedure and result of referral
Part D of the KC65 return shows for each biopsy the time elapsing before the woman is informed of the result. The NHS Cervical Screening Programme (NHSCSP) has issued guidance on waiting times, and the information is used to monitor whether clinics are meeting these standards. The return is based upon those biopsies taken during the first month of the quarter.
- Parts C1 and C2 of the KC65 return are counts of procedures undertaken at colposcopy clinics, showing the nature of treatment by result of referral. The information is used to monitor treatment patterns to ensure that treatment guidelines, such as on the number of biopsies taken, are met.
The time measured in this part of the return is the interval between the PROCEDURE DATE of the colposcopy PATIENT PROCEDURE at which the biopsy was taken and the PATIENT INFORMED BIOPSY RESULT DATE.
Total biopsies in first month of quarter
- Parts C1 and C2 are identical, except that Part C1 relates to initial treatment at first attendance, and Part C2 relates to all attendances. For part C2 data is collected on each CLINIC ATTENDANCE CONSULTANT or CLINIC ATTENDANCE NURSE in the REPORTING PERIOD.
Where a woman has a smear taken during the attendance the COLPOSCOPY PRIME PROCEDURE TYPE should be recorded as No treatment; no treatment received and no biopsy taken.
Column 2 counts the number of biopsies taken during the first month of the quarter. These are subdivided by the waiting times in lines 0001-0005.
Less than or equal to 2 weeks (line 0001)
- The procedures undertaken in the colposcopy clinics are PATIENT PROCEDURES. Only one PATIENT PROCEDURE should be counted for each CLINIC ATTENDANCE CONSULTANT or CLINIC ATTENDANCE NURSE. If more than one procedure is carried out, the most severe should be recorded for KC65.
Result of referral smear
This counts the number of women whose waiting time was less than or equal to 14 days.
>2 weeks up to 4 weeks (line 0002)
- Lines 0001 to 0008 require data on the number of women referred for colposcopy by CYTOLOGY RESULT TYPES.
Inadequate (line 0001)
This counts the number of women whose waiting time was more than 14 days but less than or equal to 28 days.
>4 weeks up to 8 weeks (line 0003)
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Inadequate sample (cat. 1).
Borderline changes (line 0002)
This counts the number of women whose waiting time was more than 28 days but less than or equal to 56 days.
>8 weeks up to 12 weeks (line 0004)
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Borderline changes (cat. 8).
Mild dyskaryosis (line 0003)
This counts the number of women whose waiting time was more than 56 days but less than or equal to 84 days.
>12 weeks (line 0005)
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Mild dyskaryosis (cat. 3).
Moderate dyskaryosis (line 0004)
This counts the number of women whose waiting time was more than 84 days.
Total (line 0006)
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Moderate dyskaryosis (cat. 7), including abnormal, unclassifiable and ungraded smears..
Severe dyskaryosis (line 0005)
This is the total for all women counted in column 2.
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Severe dyskaryosis (cat. 4).
Severe dyskaryosis/invasive carcinoma (line 0006)
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Severe dyskaryosis/invasive carcinoma (cat. 5).
Glandular neoplasia (line 0007)
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of ?Glandular neoplasia (cat. 6), including adenocarcinoma..
Referral Indication - Clinical indication (lines 0008, 0009)
- These columns count attendances for women with a REFERRAL REQUEST for colposcopy with a COLPOSCOPY REFERRAL INDICATION of Clinical indication.
Note all procedures carried out on women who have been referred to the colposcopy clinic with a REFERRAL REQUEST with a COLPOSCOPY REFERRAL INDICATION of Clinical indication should be recorded in this line regardless of the result of any smear taken after the referral.
Clinical Indication Urgent (line 0008)
- A count of the number of women with a COLPOSCOPY REFERRAL INDICATION of urgent. This is restricted to cervical lesions suspicious of cancer, or post-coital bleeding of over four weeks where the patient is aged over 35.
Clinical Indication Non-Urgent (line 0009)
- A count of the number of women with a COLPOSCOPY REFERRAL INDICATION of non-urgent. This includes all other symptomatic referrals for colposcopy
Other (line 0010)
- A count of the number of women with a CYTOLOGY RESULT TYPE classification of Other.
Entries recorded in Other (line 0010) should only occur in exceptional circumstances. NHS Cervical Screening Programme (NHSCSP) guidelines state that all smears should be identified as belonging to one of the eight recognised category classifications of CYTOLOGY RESULT TYPE. Other (line 0010) does not correspond to these recognised categories and should be used to record those rare cases in which a recognised category is not appropriate. Where an entry is present in Other (line 0010) then supporting notes should be recorded in the available box on the first page of the KC65 form.
Total (line 0011)
This is the total for all women counted in columns 2 to 8.
No treatment (column 2)
This counts the number of women who received no treatment and for whom was recorded a REFERRAL REQUEST of No treatment; no treatment received and no biopsy taken.
Procedure Type
Diagnostic biopsy (punch) (column 3)
- This counts the number of women who received no treatment and for whom a COLPOSCOPY PRIME PROCEDURE TYPE of Diagnostic biopsy (punch); no treatment received and biopsy type recorded as directed biopsy or multiple directed biopsy, or any other biopsy taken for diagnostic purposes only was recorded.
Treatment biopsy or treatment/diagnostic biopsy - Excision (column 4)
- This counts the number of women who for whom was recorded a COLPOSCOPY PRIME PROCEDURE TYPE of Loop/laser excision or knife cone; treatment method recorded as loop/laser excision or knife cone and biopsy type recorded as other than no biopsy. This will include LLETZ and NEEP.
Ablation + No Biopsy taken or biopsy result not yet known (column 5)
This counts the number of women who for whom was recorded a COLPOSCOPY PRIME PROCEDURE TYPE of Ablation; treatment method recorded as ablation. This will include cold coagulation, cryotherapy, cautery and diathermy. (ii) no biopsy taken, or biopsy result not known by clinic.
Ablation + Biopsy (column 6)
- This counts the number of women who for whom was recorded a COLPOSCOPY PRIME PROCEDURE TYPE of Ablation; treatment method recorded as ablation. This will include cold coagulation, cryotherapy, cautery and diathermy. (i) biopsy result available.
Other (column 7)
- This counts the number of women who for whom was recorded a COLPOSCOPY PRIME PROCEDURE TYPE of Other; treatment method recorded as other and biopsy type recorded as other than no biopsy. This will include polyp avulsion and treatment with silver nitrate It excludes any treatment that is not related to cervical abnormalities.
Number of first attendances (column 8)
- This is the total of all first attendances (see paragraph 2), subdivided by the CYTOLOGY RESULT TYPE classifications.
top
KC65 6
Change to Central Return Form:
Change guidance text
| Central Return Form Guidance |
KC65 - Colposcopy Clinics: Referrals, Treatments and Outcomes
KC65 - Colposcopy Clinics: Referrals, Treatments and Outcomes
Part E - Biopsies, by type and outcome
Part D - Cervical Biopsies, by time from biopsy to informing patient of result in writing
Part E of KC65 shows the histological result for each biopsy, BIOPSY REFERRAL OUTCOME, which indicates whether cancer or a pre-cancerous condition has been identified from the sample taken. The information will help to monitor whether NHS Cervical Screening Programme (NHSCSP) guidance on the quality of biopsies and accuracy of diagnosis is being met.
- Part D of the KC65 return shows for each cervical biopsy the time elapsing before the woman is informed in writing of the result. The NHS Cervical Screening Programme (NHSCSP) has issued guidance on waiting times, and the information is used to monitor whether clinics are meeting these standards. The return is based upon those biopsies taken during the first month of the quarter.
This part of the KC65 return is based upon those biopsies taken during the first month of the quarter. Please note that the total number of biopsies recorded in Part E should equal the total number recorded in Part D as both parts relate to the same biopsies.
- The time measured in this part of the return is the interval between the PROCEDURE DATE of the colposcopy PATIENT PROCEDURE at which the biopsy was taken and the PATIENT INFORMED BIOPSY RESULT DATE.
Total biopsies in first month of quarter
Column 4 counts the total number of BIOPSY REFERRAL OUTCOMES. These are analysed by biopsy type.
Biopsy Type - Diagnostic (punch) (column 2)
- Column 2 counts the number of biopsies taken during the first month of the quarter. These are subdivided by the waiting times in lines 0001-0005.
Less than or equal to 2 weeks (line 0001)
This counts the number of women for whom a COLPOSCOPY PRIME PROCEDURE TYPE of Diagnostic biopsy (punch); no treatment received and biopsy type recorded as directed biopsy or multiple directed biopsy was recorded.
Biopsy Type - Other (column 3)
- This counts the number of women whose waiting time was less than or equal to 14 days.
>2 weeks up to 4 weeks (line 0002)
This counts the number of women for whom a COLPOSCOPY PRIME PROCEDURE TYPE other than Diagnostic biopsy (punch); no treatment received and biopsy type recorded as directed biopsy or multiple directed biopsy was recorded.
Outcome (Histology result)
- This counts the number of women whose waiting time was more than 14 days but less than or equal to 28 days.
>4 weeks up to 8 weeks (line 0003)
These results are further sub-divided by BIOPSY REFERRAL OUTCOME.
Cancer (including micro-invasive) (line 0001)
- This counts the number of women whose waiting time was more than 28 days but less than or equal to 56 days.
>8 weeks up to 12 weeks (line 0004)
This counts women with a BIOPSY REFERRAL OUTCOME classification of Cancer (including micro-invasive).
Adenocarcinoma in situ (line 0002)
- This counts the number of women whose waiting time was more than 56 days but less than or equal to 84 days.
>12 weeks (line 0005)
This counts women with a BIOPSY REFERRAL OUTCOME classification of Adenocarcinoma in situ.
CIN3 (line 0003)
- This counts the number of women whose waiting time was more than 84 days.
Total (line 0006)
This counts women with a BIOPSY REFERRAL OUTCOME classification of CIN3.
CIN2 (line 0004)
This counts women with a BIOPSY REFERRAL OUTCOME classification of CIN2.
CIN1 (line 0005)
This counts women with a BIOPSY REFERRAL OUTCOME classification of CIN1.
HPV/cervicitus only (line 0006)
This counts women with a BIOPSY REFERRAL OUTCOME classification of HPV/cervicitus only.
No CIN/No HPV (line 0007)
This counts women with a BIOPSY REFERRAL OUTCOME classification of No CIN/No HPV (normal).
Inadequate biopsy (line 0008)
This counts women with a BIOPSY REFERRAL OUTCOME classification of Inadequate/unsatisfactory biopsy.
Result not known by clinic (line 0009)
This counts women with a BIOPSY REFERRAL OUTCOME classification of Result not known by clinic.
Total (line 0010)
This is the total for all women counted in columns 2, 3 and 4.
Data Quality Checks
The following data quality checks should be made:
Part D | Part E |
| | |
Column 2 Line 006 | = Column 4 Line 010 |
- This is the total for all women counted in column 2.
top
KC65 7
Change to Central Return Form:
New Form

| Central Return Form Guidance |
KC65 - Colposcopy Clinics: Referrals, Treatments and Outcomes
Part E - Cervical Biopsies, by type and outcome
Part E of KC65 shows the histological result BIOPSY REFERRAL OUTCOME for each cervical biopsy, which indicates whether cancer or a pre-cancerous condition has been identified from the sample taken. The information will help to monitor whether NHS Cervical Screening Programme (NHSCSP) guidance on the quality of biopsies and accuracy of diagnosis is being met.
This part of the KC65 return is based upon those biopsies taken during the first month of the quarter. Please note that the total number of biopsies recorded in Part E should equal the total number recorded in Part D as both parts relate to the same biopsies.
Column 5 counts the total number of BIOPSY REFERRAL OUTCOMES. These are analysed by biopsy type.
Biopsy Type - Diagnostic (punch) (column 2)
This counts the number of women for whom a COLPOSCOPY PRIME PROCEDURE TYPE of Diagnostic biopsy (punch); no treatment received and biopsy type recorded as directed biopsy or multiple directed biopsy or any other biopsy taken for diagnostic purposes only was recorded.
Biopsy Type - Excision (column 3)
This counts the number of women for whom a COLPOSCOPY PRIME PROCEDURE TYPE of Loop/laser excision or knife cone; treatment method recorded as loop/laser excision or knife cone and biopsy type recorded as other than no biopsy. This will include LLETZ and NEEP was recorded.
Biopsy Type - Other (column 3)
This counts the number of women for whom a COLPOSCOPY PRIME PROCEDURE TYPE other than Diagnostic biopsy (punch), or Loop/laser excision or knife was recorded.
Outcome (Histology result)
These results are further sub-divided by BIOPSY REFERRAL OUTCOME.
Cancer (including micro-invasive) (line 0001)
This counts women with a BIOPSY REFERRAL OUTCOME classification of Cancer (including micro-invasive).
Adenocarcinoma in situ / CGIN (line 0002)
This counts women with a BIOPSY REFERRAL OUTCOME classification of Adenocarcinoma in situ / CGIN.
CIN3 (line 0003)
This counts women with a BIOPSY REFERRAL OUTCOME classification of CIN3.
CIN2 (line 0004)
This counts women with a BIOPSY REFERRAL OUTCOME classification of CIN2.
CIN1 (line 0005)
This counts women with a BIOPSY REFERRAL OUTCOME classification of CIN1.
HPV/cervicitus only (line 0006)
This counts women with a BIOPSY REFERRAL OUTCOME classification of HPV/cervicitus only.
No CIN/No HPV (line 0007)
This counts women with a BIOPSY REFERRAL OUTCOME classification of No CIN/No HPV (normal).
Inadequate / unsatisfactory biopsy (line 0008)
This counts women with a BIOPSY REFERRAL OUTCOME classification of Inadequate/unsatisfactory biopsy.
Result not known by clinic (line 0009)
-
This counts women with a BIOPSY REFERRAL OUTCOME classification of Result not known by clinic.
Total (line 0010)
-
This is the total for all women counted in columns 2 to 5.
Data Quality Checks
-
The following data quality checks should be made:
| Part D | Part E |
| | |
| Column 2 Line 006 | = Column 4 Line 010 |
top
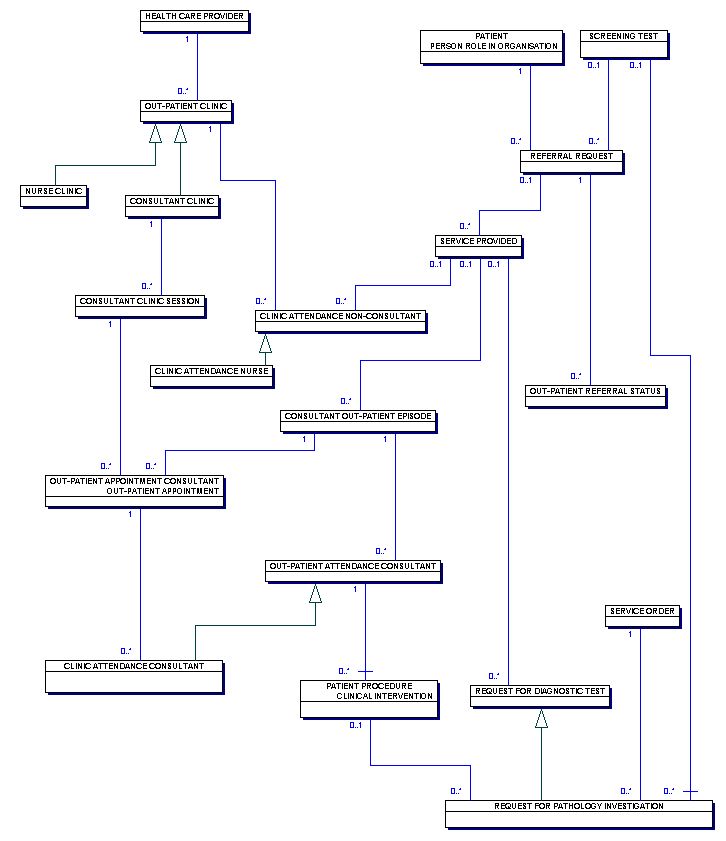
KC65 COLPOSCOPY CLINICS: REFERRALS, TREATMENTS AND OUTCOMES
Change to Diagram:
Change to diagram contents
KC65 Colposcopy Clinics: Referrals, Treatments and Outcomes
top


