NHS Connecting for Health
NHS Data Model and Dictionary Service
| Reference: | Change Request 1164 |
| Version No: | 1.0 |
| Subject: | Update Patch |
| Effective Date: | Immediate |
| Reason for Change: | Patch |
| Publication Date: | 7 June 2010 |
Background:
This patch updates the NHS Data Model and Dictionary as follows:
- Links corrected in the Commissioning Data Set overview pages
- Missing hyperlinks added
- Spelling mistakes corrected
- Html format corrected.
Summary of changes:
| Date: | 7 June 2010 |
| Sponsor: | Richard Kavanagh, NHS Connecting for Health |
Note: New text is shown with a blue background. Deleted text is crossed out. Retired text is shown in grey. Within the Diagrams deleted classes and relationships are red, changed items are blue and new items are green.
Click here for a printer friendly view of this page.
Change to Supporting Information: Changed Description
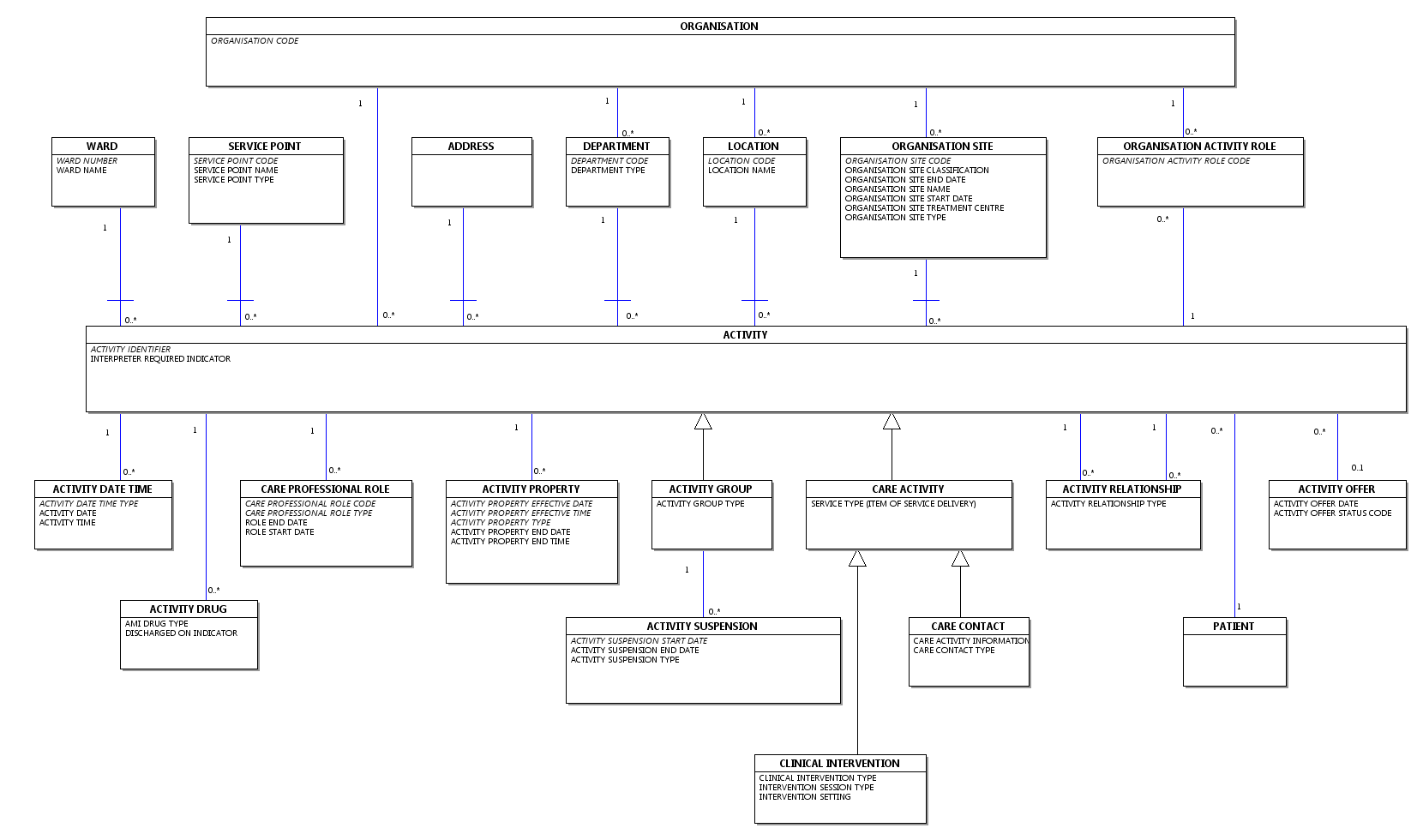
The CDS V6 Type 020 - Outpatient Commissioning Data Set carries the data for an Outpatient Attendance or a cancelled/missed APPOINTMENT.
It covers all NHS and private Outpatient ACTIVITY taking place in any:
- acute, community or mental health NHS Trust
- other NHS hospital
- non-NHS hospitals or institutions where the care delivered is NHS-funded.
under the care of a CONSULTANT, MIDWIFE or NURSE, where an appropriate MAIN SPECIALTY CODE and TREATMENT FUNCTION CODE exists.
ACTIVITY taking place under the care of Allied Health Professionals, other Biomedical Scientists and Clinical Scientists may also be carried (where an appropriate MAIN SPECIALTY CODE and TREATMENT FUNCTION CODE exists) if required although this is not a Commissioning Data Set Mandated Data Flow.
Where the Care Activity data relates to a Referral To Treatment Period Included In 18 Weeks Target, the CDS DATA GROUP : PATIENT PATHWAY data elements must be completed where appropriate.This CDS TYPE may also be used to submit Referral To Treatment Clock Stop Administrative Events.
This CDS TYPE must not be used for "Future Outpatients" - for this the CDS V6 Type 021 - Future Outpatient Commissioning Data Set must be used.
Note: CDS V6 Type 020 - Outpatient Commissioning Data Set is called Care Activity in the Commissioning Data Set XML Message Schema.
To access more detailed information on the Commissioning Data Sets, see the Commissioning Data Sets Introduction.
Data Group Overview
A high-level view of the Data Groups carried in the CDS V6 Type 021 - Future Outpatient Commissioning Data Set is shown below.A high-level view of the Data Groups carried in the CDS V6 Type 020 - Outpatient Commissioning Data Set is shown below.
See Commissioning Data Set Notation for an explanation of Group Status and Group Repeats.
| Notation | DATA GROUP OVERVIEW: CDS V6 TYPE 020 - OUTPATIENT COMMISSIONING DATA SET | ||
| Group Status | Group Repeats | FUNCTION: To support the details of an Outpatient Attendance, or a missed Appointment. | |
| M | 1..1 | DATA GROUP: CDS V6 Type 001 - Commissioning Data Set Interchange Header One per Interchange submitted to the Secondary Uses Service. Multiple Commissioning Data Set Messages may be submitted in a single Commissioning Data Set Interchange. |
| M | 1..* | DATA GROUP: CDS V6 Type 003 - Commissioning Data Set Message Header One per Commissioning Data Set Message submitted to the Secondary Uses Service. |
| M | 1..1 | DATA GROUP: CDS TRANSACTION HEADER GROUP Dependent upon the Commissioning Data Set Submission Protocol being used, one of the following must be used per Commissioning Data Set Message submitted to the Secondary Uses Service: CDS V6 Type 005B - Commissioning Data Set Transaction Header Group - Bulk Update Protocol Or CDS V6 Type 005N - Commissioning Data Set Transaction Header Group - Net Change Protocol | ||
| O | 0..1 | DATA GROUP: PATIENT PATHWAY |
| M | 1..1 | DATA GROUP: PERSON GROUP (PATIENT) | ||
| M | 1..1 | DATA GROUP: PATIENT IDENTITY | ||
| R | 0..1 | DATA GROUP: PATIENT CHARACTERISTICS (CARE ACTIVITY) | ||
| R | 0..1 | DATA GROUP: CARE EPISODE | ||
| R | 0..1 | DATA GROUP: PERSON GROUP (CONSULTANT) | ||
| O | 0..1 | DATA GROUP: CLINICAL DIAGNOSIS GROUP (ICD) | ||
| O | 0..1 | DATA GROUP: CLINICAL DIAGNOSIS GROUP (READ) | ||
| M | 1..1 | DATA GROUP: CARE ATTENDANCE | ||
| M | 1..1 | DATA GROUP: ACTIVITY CHARACTERISTICS | ||
| R | 0..1 | DATA GROUP: SERVICE AGREEMENT DETAILS | ||
| O | 0..1 | DATA GROUP: CLINICAL ACTIVITY GROUP (OPCS) | ||
| O | 0..1 | DATA GROUP: CLINICAL ACTIVITY GROUP (READ) | ||
| R | 0..1 | DATA GROUP: LOCATION GROUP - ATTENDANCE | ||
| R | 0..1 | DATA GROUP: GP REGISTRATION | ||
| R | 0..1 | DATA GROUP: CARE ACTIVITY REFERRAL | ||
| R | 0..1 | DATA GROUP: ACTIVITY CHARACTERISTICS - REFERRAL | ||
| O | 0..1 | DATA GROUP: REFERRER | ||
| R | 0..1 | DATA GROUP: MISSED APPOINTMENT OCCURRENCE | ||
| O | 0..1 | DATA GROUP: HEALTHCARE RESOURCE GROUP | ||
| M | 1..* | DATA GROUP: CDS V6 Type 004 - Commissioning Data Set Message Trailer One per Commissioning Data Set Message submitted to the Secondary Uses Service. Multiple Commissioning Data Set Messages may be submitted in a single Commissioning Data Set Interchange. |
| M | 1..1 | DATA GROUP: CDS V6 Type 002 - Commissioning Data Set Interchange Trailer One per Interchange submitted to the Secondary Uses Service. Multiple Commissioning Data Set Messages may be submitted in a single Commissioning Data Set Interchange. |
Change to Supporting Information: Changed Description
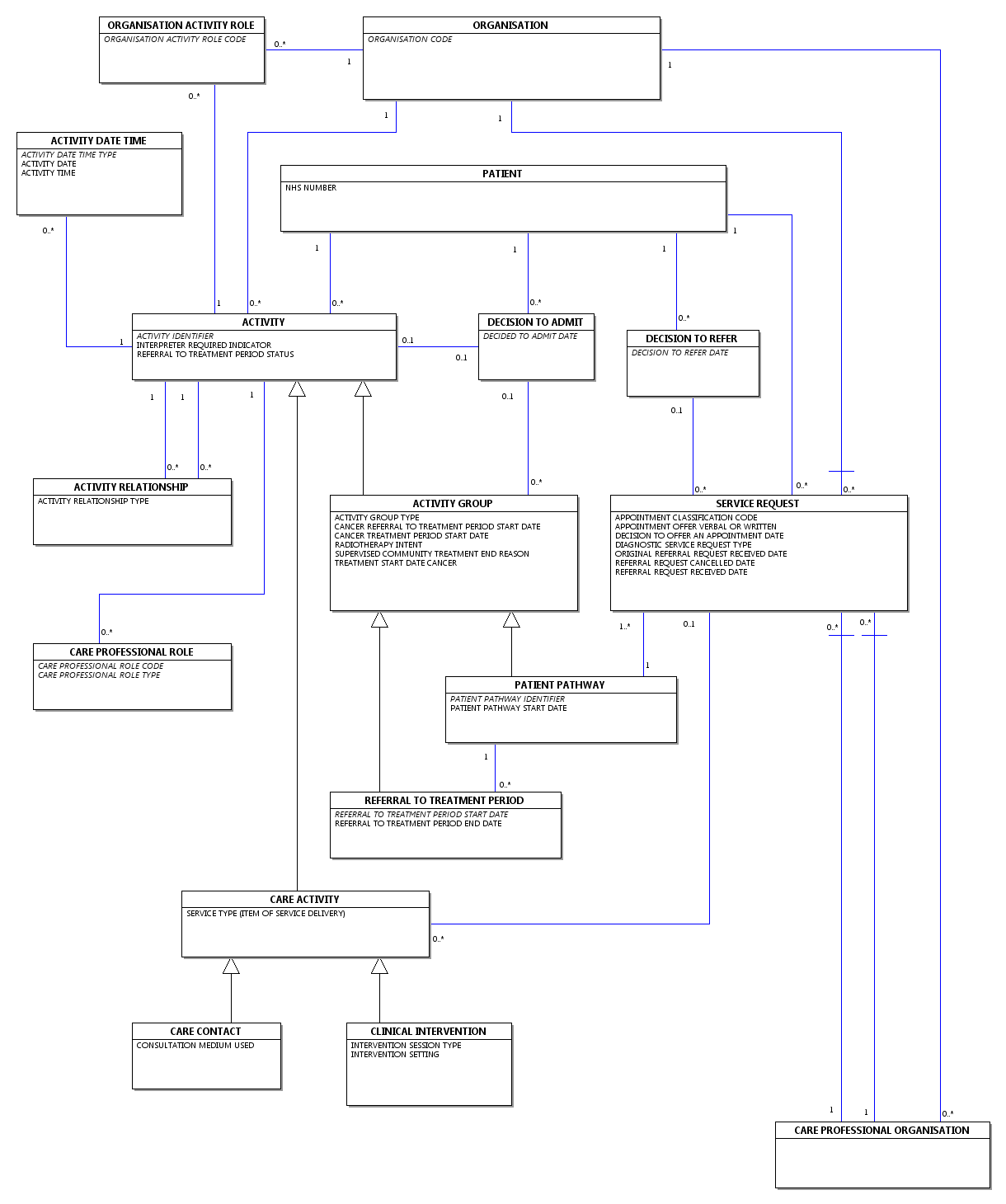
CDS V6 Type 190 - Admitted Patient Care - Unfinished General Episode Commissioning Data Set carries the data for an Unfinished General Episode.
It covers all NHS and private Admitted Patient Care (day case and inpatient) ACTIVITY taking place in any:
- acute, community or mental health NHS Trust
- other NHS hospital
- non-NHS hospitals or institutions where the care delivered is NHS-funded.
under the care of a CONSULTANT, MIDWIFE or NURSE, where an appropriate MAIN SPECIALTY CODE and TREATMENT FUNCTION CODE exists.
ACTIVITY taking place under the care of Allied Health Professionals, other Biomedical Scientists and Clinical Scientists may also be carried (where an appropriate MAIN SPECIALTY CODE and TREATMENT FUNCTION CODE exists) if required although this is not a Commissioning Data Set Mandated Data Flow.
Where the Care Activity data relates to a Referral To Treatment Period Included In 18 Weeks Target, the CDS DATA GROUP : PATIENT PATHWAY data elements must be completed where appropriate.
An Unfinished General Episode Commissioning Data Set record is required for all Unfinished General Episodes as at midnight on 31 March each year and for all unfinished short-stay informal psychiatric PATIENTS who are resident in hospital or on leave of absence (Home Leave) on 31 March and who have been in hospital for less than 12 months.
CDS V6 Type 190 - Admitted Patient Care - Unfinished General Episode Commissioning Data Set may optionally be sent more regularly, usually monthly.
To access more detailed information on the Commissioning Data Sets, see the Commissioning Data Sets Introduction.
Data Group Overview
A high-level view of the Data Groups carried in the CDS V6 Type 140 - Admitted Patient Care - Finished Delivery Episode Commissioning Data Set is shown below.A high-level view of the Data Groups carried in the CDS V6 Type 190 - Admitted Patient Care - Unfinished General Episode Commissioning Data Set is shown below.
See Commissioning Data Set Notation for an explanation of Group Status and Group Repeats.
| Notation | DATA GROUP OVERVIEW: CDS V6 TYPE 190 - APC UNFINISHED DELIVERY EPISODE COMMISSIONING DATA SET | ||
| Group Status | Group Repeats | FUNCTION: To support the details of a Finished Delivery Episode. | |
| M | 1..1 | DATA GROUP: CDS V6 Type 001 - Commissioning Data Set Interchange Header One per Interchange submitted to the Secondary Uses Service. Multiple Commissioning Data Set Messages may be submitted in a single Commissioning Data Set Interchange. |
| M | 1..* | DATA GROUP: CDS V6 Type 003 - Commissioning Data Set Message Header One per Commissioning Data Set Message submitted to the Secondary Uses Service. |
| M | 1..1 | DATA GROUP: CDS TRANSACTION HEADER GROUP Dependent upon the Commissioning Data Set Submission Protocol being used, one of the following must be used per Commissioning Data Set Message submitted to the Secondary Uses Service: CDS V6 Type 005B - Commissioning Data Set Transaction Header Group - Bulk Update Protocol Or CDS V6 Type 005N - Commissioning Data Set Transaction Header Group - Net Change Protocol | ||
| O | 0..1 | DATA GROUP: PATIENT PATHWAY |
| M | 1..1 | DATA GROUP: PERSON GROUP (PATIENT) | ||
| M | 1..1 | DATA GROUP: PATIENT IDENTITY | ||
| R | 0..1 | DATA GROUP: PATIENT CHARACTERISTICS | ||
| M | 1..1 | DATA GROUP: HOSPITAL PROVIDER SPELL | ||
| M | 1..1 | DATA GROUP: ADMISSION CHARACTERISTICS | ||
| R | 0..1 | DATA GROUP: DISCHARGE CHARACTERISTICS | ||
| M | 1..1 | DATA GROUP: CONSULTANT EPISODE | ||
| M | 1..1 | DATA GROUP: ACTIVITY CHARACTERISTICS | ||
| R | 0..1 | DATA GROUP: SERVICE AGREEMENT DETAILS | ||
| R | 0..1 | DATA GROUP: PERSON GROUP (CONSULTANT) | ||
| R | 0..1 | DATA GROUP: CLINICAL DIAGNOSIS GROUP (ICD) | ||
| O | 0..1 | DATA GROUP: CLINICAL DIAGNOSIS GROUP (READ) | ||
| R | 0..1 | DATA GROUP: CLINICAL ACTIVITY GROUP (OPCS) | ||
| O | 0..1 | DATA GROUP: CLINICAL ACTIVITY GROUP (READ) | ||
| R | 0..1 | DATA GROUP: LOCATION GROUP (AT START OF EPISODE) | ||
| O | 0..97 | DATA GROUP: LOCATION GROUP (AT WARD STAY) | ||
| O | 0..1 | DATA GROUP: LOCATION GROUP (AT END OF EPISODE) | ||
| R | 0..1 | DATA GROUP: CRITICAL CARE PERIOD | ||
| R | 0..9 | DATA GROUP: PAEDIATRIC CRITICAL CARE PERIOD | ||
| R | 0..9 | DATA GROUP: ADULT CRITICAL CARE PERIOD | ||
| R | 0..1 | DATA GROUP: GP REGISTRATION | ||
| R | 0..1 | DATA GROUP: REFERRER | ||
| R | 0..1 | DATA GROUP: ELECTIVE ADMISSION LIST ENTRY |
| O | 0..1 | DATA GROUP: HEALTHCARE RESOURCE GROUP |
| M | 1..* | DATA GROUP: CDS V6 Type 004 - Commissioning Data Set Message Trailer One per Commissioning Data Set Message submitted to the Secondary Uses Service. Multiple Commissioning Data Set Messages may be submitted in a single Commissioning Data Set Interchange. |
| M | 1..1 | DATA GROUP: CDS V6 Type 002 - Commissioning Data Set Interchange Trailer One per Interchange submitted to the Secondary Uses Service. Multiple Commissioning Data Set Messages may be submitted in a single Commissioning Data Set Interchange. |
Change to Supporting Information: Changed Description
This item is being used for development purposes and has not yet been assured by the Information Standards Board for Health and Social Care.
The data carried in the three National Service Framework for Children Young People and Maternity Services Data Sets (Maternity Data Set, National Children's and Young People's Health Services Data Set and Child and Adolescent Mental Health Services Data Set) must be structured in a particular way for submission nationally. Data items in the files, including the items in the header records, are pipe-delimited (| character).
Each data set submission file of data is structured in the following way:
- Data Set File Header Row
- Data Set Segment Row(s)
- Data Set File Trailer Row
See below for the file structure for each data set row:
- Data Set File Header Row
This contains information about the submission file, and is structured as follows, for all three data sets:
DATA SET ROW TYPE (CHILDREN YOUNG PEOPLE AND MATERNITY SERVICES)(note - always valueHDRin a file header row)YEAR AND MONTH OF REPORTING PERIODDATE AND TIME DATA SET CREATEDDATA SET IDENTIFIER (CHILDREN YOUNG PEOPLE AND MATERNITY SERVICES)DATA SET VERSION NUMBERORGANISATION CODE (CODE OF PROVIDER)The Data Set File Header row is present only once, as the first record in the submission file.
- Data Set Segment Row
This contains Segment type and PATIENT identification information followed by segment content data items, and is structured as follows:
- For the Maternity Data Set:
For the Booking and Dating Scan, Infectious Diseases and Inherited Blood Disorders, Antenatal, Labour and Delivery, and Postnatal Data Segment Groups:
DATA SET ROW TYPE (CHILDREN YOUNG PEOPLE AND MATERNITY SERVICES)(note - always valueSEGin a segment row)
DATA SET ROW TYPE (CHILDREN YOUNG PEOPLE AND MATERNITY SERVICES) (note - always value SEG in a segment row) DATA SET SEGMENT IDENTIFIER (MATERNITY DATA SET) NHS NUMBER (MOTHER) ESTIMATED DATE OF DELIVERY (AGREED) DATA SET SEGMENT IDENTIFIER (MATERNITY DATA SET)NHS NUMBER (MOTHER)ESTIMATED DATE OF DELIVERY (AGREED)For the Baby Data Segment Groups
DATA SET ROW TYPE (CHILDREN YOUNG PEOPLE AND MATERNITY SERVICES)(note - always value SEG in a segment row)DATA SET SEGMENT IDENTIFIER (MATERNITY DATA SET)NHS NUMBER (MOTHER)ESTIMATED DATE OF DELIVERY (AGREED)NHS NUMBER (BABY)
DATA SET ROW TYPE (CHILDREN YOUNG PEOPLE AND MATERNITY SERVICES) (note - always value SEG in a segment row) DATA SET SEGMENT IDENTIFIER (MATERNITY DATA SET) NHS NUMBER (MOTHER) ESTIMATED DATE OF DELIVERY (AGREED) NHS NUMBER (BABY)
For the Child and Adolescent Mental Health Services Data Set and the National Children's and Young People's Health Services Data Set:- For the Child and Adolescent Mental Health Services Data Set and the National Children's and Young People's Health Services Data Set:
DATA SET ROW TYPE (CHILDREN YOUNG PEOPLE AND MATERNITY SERVICES)(note - always valueSEGin a segment row)DATA SET SEGMENT IDENTIFIER (CHILD AND ADOLESCENT MENTAL HEALTH SERVICES DATA SET)orDATA SET SEGMENT IDENTIFIER (NATIONAL CHILDREN'S AND YOUNG PEOPLE'S HEALTH SERVICES DATA SET)NHS NUMBER
This is followed in the same row by the segment content data items, which vary depending on which segment is being submitted. This structure is repeated for each data record within the file.
- Data Set File Trailer Row
This contains some of the same information as the Data Set File Header row, plus a count of all segment records within the file, as a data quality check, and is structured as follows:
DATA SET ROW TYPE (CHILDREN YOUNG PEOPLE AND MATERNITY SERVICES)(note - always valueTRLin a file trailer row)YEAR AND MONTH OF REPORTING PERIODThe Data Set File Trailer Row is present only once, as the last record in the submission file.DATE AND TIME DATA SET CREATEDDATA SET IDENTIFIER (CHILDREN YOUNG PEOPLE AND MATERNITY SERVICES)DATA SET SEGMENT RECORDS TOTALORGANISATION CODE (CODE OF PROVIDER)
The Data Set File Trailer Row is present only once, as the last record in the submission file.Below is an example of a submission file for the Maternity Data Set:
HDR|2010-04|2010-05-31T23:34:00|MAT|1.01|RX7 (Data Set File Header Row)SEG|MAT00x|1234567890|2010-09-03|2010-04-26|2010-04-28 (Data Set Segment Row for Antenatal Admission Segment)SEG|MAT00x|0456789123|2010-04-13|5678901234|06|2010-04-14T16:06:00 (Data Set Segment Row for Critical Incident/Complications Segment)TRL|2010-04|2010-05-31T23:34:00|MAT|00002|RX7 (Data Set File Trailer Row)
| Row | Example |
|---|---|
| Data Set File Header Row | HDR|2010-04|2010-05-31T23:34:00|MAT|1.01|RX7 |
| Data Set Segment Row: Antenatal Admission | SEG|MAT00x|1234567890|2010-09-03|2010-04-26|2010-04-28 |
| Data Set Segment Row: Critical Incidents | SEG|MAT00x|0456789123|2010-04-13|5678901234|06|2010-04-14T16:06:00 |
| Data Set File Trailer Row | TRL|2010-04|2010-05-31T23:34:00|MAT|00002|RX7 |
Change to Supporting Information: Changed Description
This table details the Data Elements used in the different versions of the Commissioning Data Sets and the validation applied in each CDS TYPE.
This table is also available to download in Excel format from the CDS Supporting Information section of the NHS Data Model and Dictionary website.
Commissioning Data Set Versions
Table Structure
This table is structured with separate rows for each Data Element and separate columns for each CDS TYPE. Where the rules have changed between versions, each set of rules has its own sub-row. The cells within the body of the table show the validation applied to a Data Element for a specific CDS TYPE.
Commissioning Data Set Versions
The following notation is used in the "Version" column to identify the version or versions of the Commissioning Data Sets that the validation rule applies to.
V6-1 - CDS Version CDS006 Type List (incorporates Version CDS 6-1)
V5 - CDS Version NHS005 Type List
Where the same rules apply to several Commissioning Data Sets the first and last version are identified.
V5:6-1 Commissioning Data Set Version 5 through to Version 6-1
Where a Data Element is no longer available in a Commissioning Data Set the version number is suffixed with =R
Notation used in each table cell
Blank cell - the CDS TYPE does not include the Data Element.
Populated cell - the CDS TYPE includes the Data Element. The notation includes the content validation, optional population validation and optional additional use cases for the Data Element.
Content validation
The content validation falls into one of the following two types:
F - The format is validated, for example the format of a DATE must comply with the XML standard
V - The Data Element is validated against an explicit list of permitted values
Population validation
Where a Data Element is required, the content validation is suffixed with a population validation code:
Technical constraints
M - This Data Element is mandatory in the XML schema. Submissions will not flow if this Data Element is absent
C - There are conditions where the Data Element must be populated. In these conditions, messages will not flow if this Data Element is absent
Business constraints
R - Data required as part of NHS business rules to meet NHS business requirements. Organisations are obliged to provide this Data Element for activity provided or commissioned by the NHS.
* - There are conditions where the Data Element must not be populated. Business rules for the anonymisation of data should be applied as per the guidance issued in Security Issues and Patient Confidentiality.
Additional use cases
Secondary Uses Service business rules:
S1 This mandatory CDS DATE is used as the originating date to determine the mandatory CDS ACTIVITY DATE.
S2 The Secondary Uses Service DOES NOT support the use of the CDS TEST INDICATOR Therefore this Data Element must not be used.
S3 For Security Issues and Patient Confidentiality for further information.
S4 Used to ensure the correct sequencing of multiple and/or subsequent Commissioning Data Set submissions.
S5 These Organisation Codes must be present and registered with the Secondary Uses Service. The Commissioning Data Set Schema does not logically validate the content value of this data.
S6 All CDS REPORT PERIOD START DATES and CDS REPORT PERIOD END DATES must be consistent in all Commissioning Data Sets contained in a BULK Interchange submission.
The CDS REPORT PERIOD START DATE must be on or before the CDS REPORT PERIOD END DATE.
The CDS ACTIVITY DATE is a mandatory Data Element and must fall within the period defined. See the Commissioning Data Set Submission Protocol.
S7 See the Commissioning Data Set Addressing Grid
S8 These Data Elements are required for correct processing by the Secondary Uses Service. If omitted, the Secondary Uses Service will reject the Commissioning Data Set data.
S9 The CDS UNIQUE IDENTIFIER is a mandatory data item when using the Net Change Protocol.When using the Bulk Update Protocol this data item is optional but it is strongly advised that where it can be correctly generated and maintained it should be used/ See the Commissioning Dara Set Submission Protocol.When using the Bulk Update Protocol this data item is optional but it is strongly advised that where it can be correctly generated and maintained it should be used. See the Commissioning Data Set Submission Protocol.
S10 For CDS V6 TYPE 170 - ADMITTED PATIENT CARE - DETAINED AND/OR LONG TERM PSYCHIATRIC CENSUS COMMISSIONING DATA SET, the CDS ACTIVITY DATE contains the CDS CENSUS DATE which is also the DETAINED AND (OR) LONG TERM PSYCHIATRIC CENSUS DATE.
S11 For the following CDS TYPES, the CDS ACTIVITY DATE must contain the DATE OF ELECTIVE ADMISSION LIST CENSUS which is usually the end of the Period being reported:
CDS V6 TYPE 030 - ELECTIVE ADMISSION LIST - END OR PERIOD CENSUS (STANDARD) COMMISSIONING DATA SET
CDS V6 TYPE 040 - ELECTIVE ADMISSION LIST - END OR PERIOD CENSUS (OLD) COMMISSIONING DATA SET
CDS V6 TYPE 050 - ELECTIVE ADMISSION LIST - END OR PERIOD CENSUS (NEW) COMMISSIONING DATA SETS12These PERSON BITH DATE Data Elements mist use dates between 01/01/1880 and 31/12/2999 in order to pass validation.S12 These PERSON BIRTH DATE Data Elements must use dates between 01/01/1880 and 31/12/2999 in order to pass validation.
S13 Data Elements reporting a DATE (which is not PERSON BIRTH DATE Data Element) must use dates between 01/010/1900 and 31/12/2999 in order to pass validation.
S14 For Data Elements reporting a TIME, the hour portion must be between 00 and 23 inclusive in order to pass validation.
Healthcare Resource Groups:
H4 This Data Element is used by the Secondary Uses Service to derive Healthcare Resource Group 4. Failure to correctly populate this Data Element is likely to result in an incorrect Healthcare Resource Group, usually associated with lower levels of healthcare resource.
Additional notation
† - This notation has been applied to the following items:
CDS TYPE 021 Future Out-Patient Commissioning Data Set - Following consultation, piloting and proof that all items are appropriate, this Commissioning Data Set will be available for referrals without appointments, future scheduled appointments and cancelled appointments where the appointment date is in the future. In the interim it is recommended this CDS TYPE is only used for piloting.
Lead Care Activity Indicator - this Data Element is undefined, must not be submitted and should not flow in the Commissioning Data Sets.
LOCATION TYPE - the definition and value list for this Data Element is under review. Dependent on the review findings changes may be piloted and then approved. Until that time, this Data Element should not flow in the Commissioning Data Sets.
ADMINISTRATIVE CATEGORY (AT START OF EPISODE) and LEGAL STATUS CLASSIFICATION CODE (AT START OF EPISODE) - these Data Elements have not been piloted and therefore should not flow in the Commissioning Data Sets.
The Standard Contract
The Standard Contract Schedule 5 requires Health Care Providers to ensure that the following Commissioning Data Sets are submitted to the Commissioners on a monthly basis within 5 Operational Days of the end of the month to which the data sets relate, so that the data sets are completed by the applicable Reconciliation Point:
- Admitted Patient Care General Episode Commissioning Data Set;
- Out-patient Attendance Commissioning Data Set;
- Accident and Emergency Attendance Commissioning Data Set;
- Elective Admission List Commissioning Data Set - End of Period Census (Standard); from April 2007
- Admitted Patient Care Delivery Episode Commissioning Data Set;
- Admitted Patient Care Birth Episode Commissioning Data Set;
- Admitted Patient Care Detained / Long Term Psychiatric Census Commissioning Data Set;
- Admitted Patient Care Other Delivery Commissioning Data Set;
- Admitted Patient Care Other Birth Event Commissioning Data Set
Version | Accident and Emergency | Out-Patient | Admitted Patient Care | Elective Admission Lists | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Data Elements | 010 Accident and Emergency Attendance | 020 Out-Patient | 021 Future Out-Patient † | 120 Finished Birth | 130 Finished General | 140 Finished Delivery | 180 Unfinished Birth | 190 Unfinished General | 200 Unfinished Delivery | 150 Other Birth | 160 Other Delivery | 170 Detained and-or long term psychiatric census | 030 End of Period - Standard | 040 End of Period - Old | 050 End of Period - New | 060 End of Period - Add | 070 End of Period - Remove | 080 End of Period - Offer | 090 End of Period - Available/Unavailable | 100 End of Period - Old Service Agreement | 110 End of Period - New Service Agreement | |
| A and E ARRIVAL MODE | V5:6-1 | V R | ||||||||||||||||||||
| A and E ATTENDANCE CATEGORY | V5:6-1 | V R | ||||||||||||||||||||
| A and E ATTENDANCE CONCLUSION TIME | V5:6-1 | F R S14 | ||||||||||||||||||||
| A and E ATTENDANCE DISPOSAL | V5:6-1 | V R | ||||||||||||||||||||
| A and E ATTENDANCE NUMBER | V5:6-1 | F R | ||||||||||||||||||||
| A and E DEPARTMENT TYPE | V6:6-1 | V R | ||||||||||||||||||||
| A and E DEPARTURE TIME | V5:6-1 | F R S14 | ||||||||||||||||||||
| A and E INCIDENT LOCATION TYPE | V5:6-1 | V R | ||||||||||||||||||||
| A and E INITIAL ASSESSMENT TIME | V5:6-1 | F R S14 | ||||||||||||||||||||
| A and E PATIENT GROUP | V5:6-1 | V R | ||||||||||||||||||||
| A and E STAFF MEMBER CODE | V5:6-1 | F R | ||||||||||||||||||||
| A and E TIME SEEN FOR TREATMENT | V5:6-1 | F R S14 | ||||||||||||||||||||
| ACCIDENT AND EMERGENCY DIAGNOSIS - FIRST Known as PRIMARY DIAGNOSIS (ACCIDENT AND EMERGENCY) in the XML Schema. | V5 | F R | ||||||||||||||||||||
| V6:6-1 | F R C | |||||||||||||||||||||
| ACCIDENT AND EMERGENCY DIAGNOSIS - SECOND Known as SECONDARY DIAGNOSIS (ACCIDENT AND EMERGENCY) in the XML Schema. | V5 | F R | ||||||||||||||||||||
| V6:6-1 | F R C | |||||||||||||||||||||
| ACCIDENT AND EMERGENCY INVESTIGATION - FIRST Known as PRIMARY INVESTIGATION (ACCIDENT AND EMERGENCY) in the XML Schema. | V5 | F R | ||||||||||||||||||||
| V6:6-1 | F R C H4 | |||||||||||||||||||||
| ACCIDENT AND EMERGENCY INVESTIGATION - SECOND Known as SECONDARY INVESTIGATION (ACCIDENT AND EMERGENCY) in the XML Schema. | V5 | F R | ||||||||||||||||||||
| V6:6-1 | F R C H4 | |||||||||||||||||||||
| ACCIDENT AND EMERGENCY TREATMENT - FIRST Known as PRIMARY TREATMENT (ACCIDENT AND EMERGENCY) in the XML Schema. | V5 | F R | ||||||||||||||||||||
| V6:6-1 | F R C H4 | |||||||||||||||||||||
| ACCIDENT AND EMERGENCY TREATMENT - SECOND Known as SECONDARY TREATMENT (ACCIDENT AND EMERGENCY TREATMENT) in the XML Schema. | V5 | F R | ||||||||||||||||||||
| V6:6-1 | F R C H4 | |||||||||||||||||||||
| ACTIVITY DATE (CRITICAL CARE) | V6:6-1 | F R S13 | F R S13 | F R S13 | F R S13 | F R S13 | F R S13 | |||||||||||||||
| ADMINISTRATIVE CATEGORY | V5 | V R | V R | V R | V R | V R | V R | V R | V R | V R | V R | V R | V R | V R | ||||||||
| V6:6-1 | V R | V R | V R | V R | V R | V R | V R | |||||||||||||||
| ADMISSION METHOD (HOSPITAL PROVIDER SPELL) | V6:6-1 | V R | V R | V R | V R | V R | V R | V R | ||||||||||||||
| ADMINISTRATIVE CATEGORY (AT START OF EPISODE) This data element has not been piloted and therefore should not flow in the CDSs | V6:6-1 | †† | †† | †† | †† | †† | †† | † | ||||||||||||||
| ADMINISTRATIVE CATEGORY (ON ADMISSION) | V6:6-1 | V R | V R | V R | V R | V R | V R | V R | ||||||||||||||
| ADMISSION METHOD (HOSPITAL PROVIDER SPELL) | V5:6-1 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R | ||||||||||||||
| ADMISSION OFFER OUTCOME | V5:6-1 | V | V | V | V | V | ||||||||||||||||
| ADVANCED CARDIOVASCULAR SUPPORT DAYS | V5:6-1 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | |||||||||||||||
| ADVANCED RESPIRATORY SUPPORT DAYS | V5:6-1 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | |||||||||||||||
| AGE AT CDS ACTIVITY DATE | V6:6-1 | F M S8 | F M H4 S8 | F M S8 | F M H4 | F M H4 S8 | F M H4 | F M H4 | F M H4 S8 | F M H4 | F M H4 | F M H4 | F M | F M | F M | F M | ||||||
| AGE AT CENSUS | V5:6-1 | F M | ||||||||||||||||||||
| AGE GROUP INTENDED | V5:6-1 | V | V | V | V | V | V | V C | ||||||||||||||
| AGE ON ADMISSION | V6:6-1 | F M H4 | F M H4 | F M H4 | F M H4 | F M H4 | F M H4 | FM | ||||||||||||||
| ANAESTHETIC GIVEN DURING LABOUR OR DELIVERY | V5:6-1 | V R | V R | V R | V R | V R | V R | |||||||||||||||
| ANAESTHETIC GIVEN POST LABOUR OR DELIVERY | V5:6-1 | V R | V R | V R | V R | V R | V R | |||||||||||||||
| APPOINTMENT DATE | V5:6-1 | F R M S1 S13 | F R M S1 S13 | |||||||||||||||||||
| ARRIVAL DATE | V5:6-1 | F R M S1 S13 | ||||||||||||||||||||
| ARRIVAL TIME | V5:6-1 | F M S14 | ||||||||||||||||||||
| ATTENDANCE IDENTIFIER | V5:6-1 | F R | F R | |||||||||||||||||||
| ATTENDED OR DID NOT ATTEND Known as ATTENDANCE STATUS in the XML Schema. | V5 | V M | ||||||||||||||||||||
| V6:6-1 | V R | V R | ||||||||||||||||||||
| AUGMENTED CARE LOCATION | V5 | V | V | V | V | V | V | V | ||||||||||||||
| V6=R | ||||||||||||||||||||||
| AUGMENTED CARE OUTCOME INDICATOR | V5 | V | V | V | V | V | V | V | ||||||||||||||
| V6=R | ||||||||||||||||||||||
| AUGMENTED CARE PERIOD DISPOSAL | V5 | V | V | V | V | V | V | V | ||||||||||||||
| V6=R | ||||||||||||||||||||||
| AUGMENTED CARE PERIOD LOCAL IDENTIFIER | V5 | F | F | F | F | F | F | F | ||||||||||||||
| V6=R | ||||||||||||||||||||||
| AUGMENTED CARE PERIOD NUMBER | V5 | F | F | F | F | F | F | F | ||||||||||||||
| V6=R | ||||||||||||||||||||||
| AUGMENTED CARE PERIOD SOURCE | V5 | V | V | V | V | V | V | V | ||||||||||||||
| V6=R | ||||||||||||||||||||||
| AUGMENTED CARE PLANNED INDICATOR | V5 | V | V | V | V | V | V | V | ||||||||||||||
| V6=R | ||||||||||||||||||||||
| BASIC CARDIOVASCULAR SUPPORT DAYS | V5:6-1 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | |||||||||||||||
| BASIC RESPIRATORY SUPPORT DAYS | V5:6-1 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | |||||||||||||||
| BIRTH ORDER | V5:6-1 | F R | F R | F R | F R | F R | F R | |||||||||||||||
| BIRTH WEIGHT | V5:6-1 | F R | F R | F R | F R | F R | F R | |||||||||||||||
| CARER SUPPORT INDICATOR | V5:6-1 | V | V | V | V | V | V | V | V | V | V | V | V | V | ||||||||
| CDS ACTIVITY DATE | V6:6-1 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 | F R M S1 S13 |
| CDS APPLICABLE DATE Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 |
| CDS APPLICABLE TIME Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 |
| CDS BULK REPLACEMENT GROUP Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol Also used as a mandatory XML Attribute | V5:6-1 | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C |
| CDS CENSUS DATE Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F C S10 S13 | F C S13 | F C S13 | F C S13 | |||||||||||||||||
| CDS COPY RECIPIENT IDENTITY Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 | F S7 |
| CDS EXTRACT DATE Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 | F C S4 S13 |
| CDS EXTRACT TIME Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 | F C S4 S14 |
| CDS INTERCHANGE APPLICATION REFERENCE Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M |
| CDS INTERCHANGE CONTROL COUNT Commissioning Data Set 'Trailer' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M |
| CDS INTERCHANGE CONTROL REFERENCE Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M |
| CDS INTERCHANGE DATE OF PREPARATION Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 | F M S13 |
| CDS INTERCHANGE RECEIVER IDENTITY Commissioning Data Set 'Header' and 'Trailer' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 |
| CDS INTERCHANGE SENDER IDENTITY Commissioning Data Set 'Header' and 'Trailer' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 |
| CDS INTERCHANGE TEST INDICATOR Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V | V |
| CDS INTERCHANGE TIME OF PREPARATION Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 | F M S14 |
| CDS MESSAGE REFERENCE Commissioning Data Set 'Header' and 'Trailer' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M |
| CDS MESSAGE TYPE Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol Also used as a mandatory XML Attribute | V5:6-1 | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M |
| CDS MESSAGE VERSION NUMBER Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M |
| CDS PRIME RECIPIENT IDENTITY Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V6:6-1 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 | F M S5 S7 |
| CDS PROTOCOL IDENTIFIER Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol Also used as a mandatory XML Attribute | V5:6-1 | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M | F M |
| CDS REPORT PERIOD START DATE Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 |
| CDS REPORT PERIOD END DATE Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 | F C S6 S13 |
| CDS SENDER IDENTITY Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V6:6-1 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 | F M S5 |
| CDS TEST INDICATOR Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 | F S2 |
| CDS TYPE Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol Also used as a mandatory XML attribute | V5:6-1 | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M |
| CDS UNIQUE IDENTIFIER This is a mandatory data item when using the Net Change Protocol. When using the Bulk Update Protocol this data item is optional but it is strongly advised that where it can be correctly generated and maintained it should be used. See Commissioning Data Set Submission Protocol. | V5:6-1 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 | F C S9 |
| CDS UPDATE TYPE Commissioning Data Set 'Header' Data Item, mandatory dependent upon Bulk or Net Protocol | V5:6-1 | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C |
| COMMISSIONER REFERENCE NUMBER | V5:6-1 | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | ||
| COMMISSIONING SERIAL NUMBER | V5:6-1 | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | |||
| CONSULTANT CODE | V5:6-1 | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | ||||||||
| COUNT OF DAYS SUSPENDED | V5:6-1 | F R | F R | F R | F R | |||||||||||||||||
| CRITICAL CARE ACTIVITY CODE | V6:6-1 | V R | V R | V R | V R | V R | V R | |||||||||||||||
| CRITICAL CARE ADMISSION SOURCE | V6:6-1 | V | V | V | V | V | V | |||||||||||||||
| CRITICAL CARE ADMISSION TYPE | V6:6-1 | V | V | V | V | V | V | |||||||||||||||
| CRITICAL CARE DISCHARGE DATE | V5:6-1 | F R H4 S13 | F R H4 S13 | F R H4 S13 | F R H4 S13 | F R H4 S13 | F R H4 S13 | |||||||||||||||
| CRITICAL CARE DISCHARGE DESTINATION | V6:6-1 | V | V | V | V | V | V | |||||||||||||||
| CRITICAL CARE DISCHARGE LOCATION | V6:6-1 | V | V | V | V | V | V | |||||||||||||||
| CRITICAL CARE DISCHARGE READY DATE | V6:6-1 | F S13 | F S13 | F S13 | F S13 | F S13 | F S13 | |||||||||||||||
| CRITICAL CARE DISCHARGE READY TIME | V6:6-1 | F S14 | F S14 | F S14 | F S14 | F S14 | F S14 | |||||||||||||||
| CRITICAL CARE DISCHARGE STATUS | V6:6-1 | V | V | V | V | V | V | |||||||||||||||
| CRITICAL CARE DISCHARGE TIME | V5:6-1 | F R S14 | F R S14 | F R S14 | F R S14 | F R S14 | F R S14 | |||||||||||||||
| CRITICAL CARE LEVEL 2 DAYS | V5:6-1 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | |||||||||||||||
| CRITICAL CARE LEVEL 3 DAYS | V5:6-1 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | |||||||||||||||
| CRITICAL CARE LOCAL IDENTIFIER | V5:6-1 | F R | F R | F R | F R | F R | F R | |||||||||||||||
| CRITICAL CARE SOURCE LOCATION | V6:6-1 | V | V | V | V | V | V | |||||||||||||||
| CRITICAL CARE START DATE | V5:6-1 | F R H4 S13 | F R H4 S13 | F R H4 S13 | F R H4 S13 | F R H4 S13 | F R H4 S13 | |||||||||||||||
| CRITICAL CARE START TIME | V6:6-1 | F R C S14 | F R C S14 | F R C S14 | F R C S14 | F R C S14 | F R C S14 | |||||||||||||||
| CRITICAL CARE UNIT BED CONFIGURATION | V6:6-1 | V | V | V | V | V | V | |||||||||||||||
| CRITICAL CARE UNIT FUNCTION | V5:6-1 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | |||||||||||||||
| DATE DETENTION COMMENCED | V5:6-1 | F R S13 | ||||||||||||||||||||
| DECIDED TO ADMIT DATE | V5:6-1 | F R S13 | F R S13 | F R S13 | F S1 S13 | F S13 | F S13 | F S13 | ||||||||||||||
| DELIVERY DATE | V5:6-1 | F R S13 | F R S13 | F R S13 | F R S13 | F S1 S13 | F S1 S13 | |||||||||||||||
| DELIVERY METHOD | V5:6-1 | V R | V R | V R | V R | V R | V R | |||||||||||||||
| DELIVERY PLACE CHANGE REASON | V5:6-1 | V R | V R | V R | V R | V R | V R | |||||||||||||||
| DELIVERY PLACE TYPE (ACTUAL) | V5:6-1 | V R | V R | V R | V R | V R | V R | |||||||||||||||
| DELIVERY PLACE TYPE (INTENDED) | V5:6-1 | V R | V R | V R | V R | V R | V R | |||||||||||||||
| DERMATOLOGICAL SUPPORT DAYS | V5:6-1 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | |||||||||||||||
| DETAINED AND (OR) LONG TERM PSYCHIATRIC CENSUS DATE | V5:6-1 | F C S1 S10 S13 | ||||||||||||||||||||
| DIAGNOSIS SCHEME IN USE | V5:6-1 | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | |||||||||||
| DISCHARGE DATE (HOSPITAL PROVIDER SPELL) | V6:6-1 | F R S13 | F R S13 | F R S13 | F S13 | F S13 | F S13 | |||||||||||||||
| DISCHARGE DESTINATION (HOSPITAL PROVIDER SPELL) | V5:6-1 | V R H4 | V R H4 | V R H4 | V H4 | V H4 | V H4 | |||||||||||||||
| DISCHARGE METHOD (HOSPITAL PROVIDER SPELL) | V5:6-1 | V R H4 | V R H4 | V R H4 | V H4 | V H4 | V H4 | |||||||||||||||
| DISCHARGE READY DATE (HOSPITAL PROVIDER SPELL) | V6:6-1 | F R S13 | F R S13 | F R S13 | F S13 | F S13 | F S13 | |||||||||||||||
| DURATION OF CARE TO PSYCHIATRIC CENSUS DATE | V5:6-1 | F R S13 | ||||||||||||||||||||
| DURATION OF DETENTION | V5:6-1 | F R | ||||||||||||||||||||
| DURATION OF ELECTIVE WAIT | V5:6-1 | F R | F R | F R | ||||||||||||||||||
| EARLIEST REASONABLE OFFER DATE | V6:6-1 | F S13 | F S13 | F S13 | F S13 | F S13 | F S13 | F S13 | F S13 | F S13 | F S13 | |||||||||||
| ELECTIVE ADMISSION LIST ENTRY NUMBER | V5:6-1 | F R | F R | F R | F R | |||||||||||||||||
| ELECTIVE ADMISSION LIST REMOVAL DATE | V5:6-1 | F S13 | F S13 | F S13 | F S1 S13 | |||||||||||||||||
| ELECTIVE ADMISSION LIST REMOVAL REASON | V5:6-1 | V | V | V | ||||||||||||||||||
| ELECTIVE ADMISSION LIST STATUS | V5:6-1 | V R | V R | V R | V R | |||||||||||||||||
| ELECTIVE ADMISSION TYPE | V5:6-1 | V R | V R | V R | V R | |||||||||||||||||
| END DATE (EPISODE) | V5:6-1 | F M S1 H4 S13 | F M S1 H4 S13 | F M S1 H4 S13 | F S13 | F S13 | F S13 | |||||||||||||||
| END DATE | V5:6-1 | F S13 | F S13 | F S13 | F S13 | F S13 | F S13 | |||||||||||||||
| EPISODE NUMBER | V5:6-1 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | F R | ||||||||||||||
| ETHNIC CATEGORY (Note this item has not been approved by ISB for CDS Type 021, but is included as a placeholder for future development.) | V5:6 | V | V R | V R | V | V R | V R | V | V R | V R | ||||||||||||
| V6-1 | V R | V R | V | V R | V R | V R | V R | V R | V R | V R | V R | V R | ||||||||||
| FIRST ATTENDANCE | V5:6-1 | V R H4 | V R | |||||||||||||||||||
| FIRST ANTENATAL ASSESSMENT DATE | V5:6-1 | F R S13 | F R S13 | F R S13 | F R S13 | F R S13 | F R S13 | |||||||||||||||
| FIRST REGULAR DAY OR NIGHT ADMISSION | V5:6-1 | V | V | |||||||||||||||||||
| GASTRO-INTESTINAL SUPPORT DAYS | V6:6-1 | F | F | F | F | F | F | |||||||||||||||
| GENERAL MEDICAL PRACTITIONER (ANTENATAL CARE) (formerly GMP (CODE OF GMP RESPONSIBLE FOR ANTENATAL CARE)) | V5:6-1 | F R | F R | F R | F R | F R | F R | |||||||||||||||
| GENERAL MEDICAL PRACTITIONER (SPECIFIED) (formerly GMP (CODE OF REGISTERED OR REFERRING GMP)) | V5:6-1 | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | |||||
| GENERAL MEDICAL PRACTITIONER PRACTICE (ANTENATAL CARE) (formerly CODE OF GP PRACTICE (REGISTERED GMP - ANTENATAL CARE)) | V5:6-1 | F | F | F | F | F | F | |||||||||||||||
| GENERAL MEDICAL PRACTICE CODE (PATIENT REGISTRATION) (formerly CODE OF GP PRACTICE (REGISTERED GMP)) | V5:6-1 | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | |||||
| GESTATION LENGTH (ASSESSMENT) | V5:6-1 | V R | V R | V R | V R | V | V | |||||||||||||||
| GESTATION LENGTH (AT DELIVERY) | V6:6-1 | V R | V R | V R | V R | |||||||||||||||||
| GESTATION LENGTH (LABOUR ONSET) | V5:6-1 | V | V R | V R | V R | V | V | |||||||||||||||
| GUARANTEED ADMISSION DATE | V5:6-1 | F S13 | F S13 | F S13 | F | |||||||||||||||||
| HEALTHCARE RESOURCE GROUP CODE | V5:6-1 | F R | F R | F | F R | F R | F R | F R | F R | F R | F R | F | F | F | F | |||||||
| HEALTHCARE RESOURCE GROUP CODE-VERSION NUMBER | V5:6-1 | F R | F R | F | F R | F R | F R | F R | F R | F R | F R | F | F | F | F | |||||||
| HIGH COST DRUGS (OPCS) | V6:6-1 | F R | F R | F R | F R | F R | F R | |||||||||||||||
| HIGH DEPENDENCY CARE LEVEL DAYS | V5 | F | F | F | F | F | F | |||||||||||||||
| V6=R | ||||||||||||||||||||||
| HOSPITAL PROVIDER SPELL NUMBER | V5:6-1 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | F R | ||||||||||||||
| HRG DOMINANT GROUPING VARIABLE-PROCEDURE | V5:6-1 | F | F | F | F R | F R | F R | F R | F R | F R | F R | F | F | F | F | |||||||
| INTENDED CLINICAL CARE INTENSITY | V5:6-1 | V | V | V | V | V | V | V | ||||||||||||||
| INTENDED MANAGEMENT | V5:6-1 | V R | V R | V R | V R | V R | V R | V R | ||||||||||||||
| INTENDED PROCEDURE (OPCS) | V5:6-1 | F | F | F | F | |||||||||||||||||
| INTENDED PROCEDURE (READ) | V5:6-1 | F | F | F | F | |||||||||||||||||
| INTENDED PROCEDURE STATUS | V5:6-1 | V R | V R | V R | V R | |||||||||||||||||
| INTENDED SITE CODE (OF TREATMENT) | V5:6-1 | F | F | F | F | |||||||||||||||||
| INTENSIVE CARE LEVEL DAYS | V5 | F | F | F | F | F | F | |||||||||||||||
| V6=R | ||||||||||||||||||||||
| INVESTIGATION SCHEME IN USE | V5:6-1 | V C | ||||||||||||||||||||
| LABOUR OR DELIVERY ONSET METHOD | V5:6-1 | V R | V R | V R | V R | V R | V R | |||||||||||||||
| LAST DNA OR PATIENT CANCELLED DATE | V5:6-1 | F R S13 | F R S13 | F R S13 | F R S13 | F R S13 | F R | |||||||||||||||
| LAST EPISODE IN SPELL INDICATOR | V5:6-1 | V R | V R | V R | V R | V R | V R | |||||||||||||||
| LEAD CARE ACTIVITY INDICATOR This data element is undefined, must not be submitted and should not flow in the CDSs | V6:6-1 | †† | †† | †† | †† | †† | †† | †† | †† | †† | †† | †† | †† | †† | †† | †† | †† | †† | †† | †† | †† | †† |
| LEGAL STATUS CLASSIFICATION CODE (AT CENSUS DATE) | V5:6-1 | V R S13 | ||||||||||||||||||||
| LEGAL STATUS CLASSIFICATION CODE (AT START OF EPISODE) This data element has not been piloted and therefore should not flow in the CDSs | V6:6-1 | ††† | ††† | ††† | ††† | |||||||||||||||||
| LEGAL STATUS CLASSIFICATION CODE (ON ADMISSION) | V5:6-1 | V R | V R | V R | V C | V R | ||||||||||||||||
| LIVE OR STILL BIRTH | V5:6-1 | V R | V R | V R | V R | V R | V R | |||||||||||||||
| LIVER SUPPORT DAYS | V5:6-1 | F R | F R | F R | F R | F R | F R | |||||||||||||||
| LOCAL PATIENT IDENTIFIER* | V5:6-1 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | |||||
| LOCAL PATIENT IDENTIFIER (BABY)* | V5:6-1 | F C S3 | F C S3 | F C S3 | ||||||||||||||||||
| LOCAL PATIENT IDENTIFIER (MOTHER)* | V5:6-1 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | ||||||||||||||||
| LOCATION CLASS | V5:6-1 | V R | V R | V R | V R | V R | V R | V R | V R | V R | V R | V R | V | V | V | V | ||||||
| LOCATION TYPE The definition and value list for this data element is under review. Dependent on the review findings changes may be piloted and then approved. Until that time this data element should not flow in the CDSs. | V6:6-1 | ††† | ††† | ††† | ††† | † | ††† | ††† | ††† | ††† | ††† | ††† | ††† | ††† | ††† | ††† | ||||||
| MAIN SPECIALTY CODE | V5:6-1 | V R H4 | V R | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R | V R | V R | V R | V R | ||||||
| MEDICAL STAFF TYPE SEEING PATIENT | V5:6-1 | V R | V R | |||||||||||||||||||
| MENTAL CATEGORY (for patients detained before 1 October 2008) | V5:6-1 | V R | ||||||||||||||||||||
| MENTAL HEALTH ACT 2007 MENTAL CATEGORY (for patients detained after 30 September 2008) | V6-1 | V R | ||||||||||||||||||||
| NEONATAL LEVEL OF CARE | V5:6-1 | V H4 | V H4 | V H4 | V H4 | |||||||||||||||||
| NEUROLOGICAL SUPPORT DAYS | V5:6-1 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | |||||||||||||||
| NHS NUMBER* | V5:6-1 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | F C S3 | |||||
| NHS NUMBER (BABY)* | V5:6-1 | F C S3 | F C S3 | F C S3 | ||||||||||||||||||
| NHS NUMBER (MOTHER)* | V5:6-1 | F C S3 | F C S3 | F C S3 | ||||||||||||||||||
| NHS NUMBER STATUS INDICATOR | V5:6-1 | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | V M | |||||
| NHS NUMBER STATUS INDICATOR (BABY) | V5:6-1 | V C | V C | VC | ||||||||||||||||||
| NHS NUMBER STATUS INDICATOR (MOTHER) | V5:6-1 | V C | V C | V C | ||||||||||||||||||
| NHS SERVICE AGREEMENT CHANGE DATE | V5:6-1 | F R S1 S13 | F S1 S13 | F S1 S13 | F R S1 S13 | F S13 | F S1 S13 | F S1 S13 | ||||||||||||||
| NHS SERVICE AGREEMENT LINE NUMBER | V5:6-1 | F | F | F | F | F | F | F | F | F | F | F | F | F | F | F | F | F | F | F | F | F |
| NUMBER OF AUGMENTED CARE PERIODS WITHIN EPISODE | V5 | F | F | F | F | F | F | |||||||||||||||
| V6-1=R | ||||||||||||||||||||||
| NUMBER OF BABIES | V5:6-1 | V R | V R | V R | V R | V R | V R | |||||||||||||||
| NUMBER OF ORGAN SYSTEMS SUPPORTED | V5 | F | F | F | F | F | F | |||||||||||||||
| V6-1=R | ||||||||||||||||||||||
| OFFERED FOR ADMISSION DATE | V5:6-1 | F R S13 | F R S13 | F R S13 | F M S1 S13 | F R S13 | ||||||||||||||||
| OPERATION STATUS | V5:6-1 | V | V | V R | V R | V R | V R | V R | V R | |||||||||||||
| ORGANISATION CODE (LOCAL PATIENT IDENTIFIER) | V5:6-1 | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | |||||
| ORGANISATION CODE (LOCAL PATIENT IDENTIFIER (BABY)) | V5:6-1 | F C | F C | F C | ||||||||||||||||||
| ORGANISATION CODE (LOCAL PATIENT IDENTIFIER (MOTHER)) | V5:6-1 | F C | F C | F C | ||||||||||||||||||
| ORGANISATION CODE (PATIENT PATHWAY IDENTIFIER ISSUER) | V6:6-1 | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C |
| ORGANISATION CODE (PCT OF RESIDENCE) | V5:6-1 | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | ||||
| ORGANISATION CODE (PCT OF RESIDENCE (MOTHER)) | V6:6-1 | F R | F R | F R | ||||||||||||||||||
| ORGANISATION CODE (CODE OF COMMISSIONER) Numeric Validation is applied in the Schema | V5:6-1 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | |||
| ORGANISATION CODE (CODE OF PROVIDER) | V5:6-1 | F R S8 | F R S8 | F R S8 | F R H4 S8 | F R H4 S8 | F R H4 S8 | F R H4 S8 | F R H4 S8 | F R H4 S8 | F R H4 S8 | F R H4 S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | F R S8 | |||
| ORGAN SUPPORT MAXIMUM | V6:6-1 | V | V | V | V | V | V | |||||||||||||||
| ORIGINAL DECIDED TO ADMIT DATE | V5:6-1 | F R S13 | F R S13 | F R S13 | F R S13 | |||||||||||||||||
| OUTCOME OF ATTENDANCE | V5:6-1 | V R | V | |||||||||||||||||||
| PATIENT CLASSIFICATION | V5:6-1 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R | ||||||||||||||
| PATIENT NAME | V5:6-1 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | |||||
| PATIENT PATHWAY IDENTIFIER | V6:6-1 | F C | F R | F C | F C | F R | F C | F C | F R | F C | F C | F C | F C | F R | F C | F C | F R | F R | F R | F C | F C | F C |
| PATIENT USUAL ADDRESS* | V5:6-1 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | |||||
| PATIENT USUAL ADDRESS (MOTHER)* | V5:6-1 | F S3 | F S3 | F S3 | ||||||||||||||||||
| PERSON BIRTH DATE* | V6:6-1 | F R S3 S12 | F R S3 S12 | F R S3 S12 | F R S3 S12 | F R S3 S12 | F R S3 S12 | F R S3 S12 | F R S3 S12 | F R S3 S12 | F R S3 S12 | F R S3 S12 | F R S3 S12 | F R S3 S12 | F R S3 S12 | F R S3 S12 | F R S3 S12 | |||||
| PERSON BIRTH DATE (BABY)* | V6:6-1 | F R S3 S12 | F R S3 S12 | F R S3 S12 | ||||||||||||||||||
| PERSON BIRTH DATE (MOTHER)* | V6:6-1 | F R S3 S12 | F R S3 S12 | F R S3 S12 | ||||||||||||||||||
| PERSON GENDER CURRENT | V5:6-1 | V R | V R H4 | V R | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R | V R | V R | V R | V R | |||||
| PERSON GENDER CURRENT (BABY) | V5:6-1 | V R | V R | V R | ||||||||||||||||||
| PERSON MARITAL STATUS* | V5:6-1 | V C | V C | V C | V C | V C | V C | |||||||||||||||
| PERSON WEIGHT | V6:6-1 | F R | F R | F R | F R | |||||||||||||||||
| POSTCODE OF USUAL ADDRESS* | V5:6-1 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | F S3 | |||||
| POSTCODE OF USUAL ADDRESS (MOTHER)* | V5:6-1 | F S3 | F S3 | F S3 | ||||||||||||||||||
| PREGNANCY TOTAL PREVIOUS PREGNANCIES | V5:6-1 | V R | V R | V R | ||||||||||||||||||
| PRIMARY DIAGNOSIS (ICD) | V5:6-1 | F | F C | F C | F C H4 | F C H4 | F C H4 | F C H4 | F C H4 | F C H4 | F C | |||||||||||
| PRIMARY DIAGNOSIS (READ) | V5:6-1 | F | F C | F C | F C | F C | F C | F C | F C | F C | F C | |||||||||||
| PRIMARY PROCEDURE (OPCS) | V5:6-1 | F C | F C H4 | F C H4 | F C H4 | F C H4 | F C H4 | F C H4 | F C H4 | F C H4 | F C | F C | F C | F C | F C | |||||||
| PRIMARY PROCEDURE (READ) | V5:6-1 | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | |||||||
| PRIORITY TYPE | V5:6-1 | V R | V R | V R | V R | V R | V R | |||||||||||||||
| PROCEDURE (OPCS) Known as SECONDARY PROCEDURE (OPCS) in the XML Schema. | V5 | F C | F C | F C | F C | F C | F C | |||||||||||||||
| V6:6-1 | F | F H4 | F | F C H4 | F C H4 | F C H4 | F C H4 | F C H4 | F C H4 | F | F | F | F | |||||||||
| PROCEDURE (READ) Known as SECONDARY PROCEDURE (READ) in the XML Schema. | V5 | F C | F C | F C | F C | F C | F C | |||||||||||||||
| V6:6-1 | F | F | F | F | F | F | F | F | F | F | F | F | F | |||||||||
| PROCEDURE DATE | V5:6-1 | F C S13 | F C S13 | F C S13 | F C S13 | F C S13 | F C S13 | F C S13 | F C S13 | F C S13 | F C S13 | F C S13 | F C S13 | F C S13 | ||||||||
| PROCEDURE SCHEME IN USE | V5:6-1 | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | ||||||||
| PROVIDER REFERENCE NUMBER | V5:6-1 | F | F | F | F | F | F | F | F | F | F | F | F | F | F | F | F | F | F | |||
| PSYCHIATRIC PATIENT STATUS | V5:6-1 | V R | V R | V R | V R | V R | ||||||||||||||||
| REFERRAL REQUEST RECEIVED DATE | V5:6-1 | F R S13 | F R S13 | |||||||||||||||||||
| REFERRAL TO TREATMENT PERIOD END DATE | V6:6-1 | F S13 | F R S13 | F S13 | F S13 | F R S13 | F S13 | F S13 | F R S13 | F S13 | F S13 | F S13 | F S13 | F R S13 | F S13 | F S13 | F R S13 | F R S13 | F R S13 | F S13 | F S13 | F S13 |
| REFERRAL TO TREATMENT PERIOD START DATE | V6:6-1 | F S13 | F R S13 | F S13 | F S13 | F R S13 | F S13 | F S13 | F R S13 | F S13 | F S13 | F S13 | F S13 | F R S13 | F S13 | F S13 | F R S13 | F R S13 | F R S13 | F S13 | F S13 | F S13 |
| REFERRAL TO TREATMENT STATUS | V6:6-1 | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C | V C |
| REFERRER CODE | V5:6-1 | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | ||||||||
| REFERRING ORGANISATION CODE | V5:6-1 | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | F R | ||||||||
| RENAL SUPPORT DAYS | V5:6-1 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | F R H4 | |||||||||||||||
| RESUSCITATION METHOD | V5:6-1 | V R | V R | V R | V R | V R | V R | |||||||||||||||
| SECONDARY DIAGNOSIS (ICD) | V5:6-1 | F C | F C | F C | F C H4 | F C H4 | F C H4 | F C H4 | F C H4 | F C H4 | F | |||||||||||
| SECONDARY DIAGNOSIS (READ) | V5:6-1 | F C | F C | F C | F C | F C | F C | F C | F C | F C | F | |||||||||||
| SERVICE TYPE REQUESTED | V5:6-1 | V R | V R | |||||||||||||||||||
| SEX OF PATIENTS | V5:6-1 | V | V | V | V | V | V | V | ||||||||||||||
| SITE CODE (OF TREATMENT) | V5:6-1 | F R | F | F R | F R | F R | F R | F R | F R | F R | ||||||||||||
| SOURCE OF ADMISSION (HOSPITAL PROVIDER SPELL) | V5:6-1 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R | ||||||||||||||
| SOURCE OF REFERRAL FOR A and E | V5:6-1 | V R | ||||||||||||||||||||
| SOURCE OF REFERRAL FOR OUT-PATIENTS | V5:6-1 | V R | V R | |||||||||||||||||||
| START DATE (EPISODE) | V5:6-1 | F M H4 S13 | F M H4 S13 | F M H4 S13 | F M H4 S1 S13 | F M H4 S1 S13 | F M H4 S1 S13 | F M S13 | ||||||||||||||
| START DATE (HOSPITAL PROVIDER SPELL) | V5:6-1 | F M H4 S13 | F M H4 S13 | F M H4 S13 | F M H4 S13 | F M H4 S13 | F M H4 S13 | F M S13 | ||||||||||||||
| START DATE | V5:6-1 | F S13 | F S13 | F S13 | F S13 | F S13 | F S13 | |||||||||||||||
| STATUS OF PATIENT INCLUDED IN THE PSYCHIATRIC CENSUS | V5:6-1 | V R | ||||||||||||||||||||
| STATUS OF PERSON CONDUCTING DELIVERY | V5:6-1 | V R | V R | V R | V R | V R | V R | |||||||||||||||
| SUSPENSION END DATE | V5:6-1 | F R S13 | ||||||||||||||||||||
| SUSPENSION START DATE | V5:6-1 | F S1 S13 | ||||||||||||||||||||
| TREATMENT FUNCTION CODE | V5:6-1 | V R H4 | V R | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R H4 | V R | V R | V R | V R | V R | ||||||||
| UNIQUE BOOKING REFERENCE NUMBER (CONVERTED) | V6:6-1 | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C | F C |
| WAITING LIST ENTRY LAST REVIEWED DATE | V5:6-1 | F S13 | F S13 | F S13 | F S13 | |||||||||||||||||
| WARD DAY PERIOD AVAILABILITY | V5:6-1 | V | V | V | V | V | V | V | ||||||||||||||
| WARD NIGHT PERIOD AVAILABILITY | V5:6-1 | V | V | V | V | V | V | V | ||||||||||||||
Change to Class: Changed Attributes
| A and E ARRIVAL MODE | ||
| A and E ATTENDANCE CATEGORY | ||
| A and E ATTENDANCE DISPOSAL | ||
| A and E INITIAL ASSESSMENT TRIAGE CATEGORY | ||
| A and E STREAM | ||
| ACUTE HOME-BASED TELEPHONE CONTACT | ||
| ANTENATAL OR POSTNATAL INDICATOR | ||
| BREAST CANCER NURSE SEEN | ||
| CARE ACTIVITY INFORMATION | ||
| CARE CONTACT TYPE | ||
| COLPOSCOPY PRIME PROCEDURE TYPE | ||
| CONSULTATION MEDIUM USED | ||
| CONTRACEPTIVE SERVICE TYPE | ||
| CPA REVIEW OUTCOME | ||
| DENTAL HAEMORRHAGE SERVICE TYPE | ||
| DENTAL REFERRAL INDICATOR | ||
| ELIGIBILITY OUTCOME | ||
| EMERGENCY TREATMENT FEE | ||
| EMERGENCY TREATMENT TYPE | ||
| FIRST ATTENDANCE | ||
| GENITOURINARY CONTACT TYPE CODE | ||
| HEALTH PROMOTION STAFF GROUP | ||
| HOME HELP USE | ||
| INITIAL CONTACT | ||
| INITIAL CONTACT WITHIN FIVE DAYS | ||
| IUD APPLICATION DATE | ||
| MARKER RESPONSE STATUS | ||
| MATERNITY MEDICAL SERVICE TYPE | ||
| MATERNITY VISIT CALL REASON | ||
| MEDICAL STAFF TYPE SEEING PATIENT | ||
| METASTATIC STATUS | ||
| NODAL STATUS | ||
| NON-NHS COMMUNITY BED USE | ||
| NON-NHS DAY CARE FACILITY USE | ||
| OUTCOME OF ATTENDANCE | ||
| PATIENT INFORMED OF OUTCOME DATE | ||
| PATIENT REPORTED SYMPTOMS INDICATOR | ||
| PATIENT REPORTED WAIT | ||
| PATIENT TRIAL STATUS | ||
| PAYMENT FROM PATIENT RECEIVED | ||
| POSTNATAL CARE INDICATOR | ||
| PRIMARY TUMOUR STATUS | ||
| SETTLED ACCOMMODATION INDICATOR | ||
| SHELTERED WORK FACILITY USE | ||
| SIGHT TEST DOMICILIARY VISIT TYPE | ||
| SIGHT TEST FORM COMPLETED | ||
| SIGHT TEST PERSON SUBSIDY TYPE | ||
| SKIN TUMOUR STATUS | ||
| STATUTORY ASSESSMENT TYPE | ||
| SURVEILLANCE RESULT |
Change to Attribute: Changed Description
A coded CLINICAL INVESTIGATION RESULT ITEM for a Clinical Investigation of a Newborn Hearing Screening.
National Codes:
| 01 | Clear response, no follow up required |
| 02 | Clear response, targeted follow up required |
| 03 | No clear response, bilateral referral |
| 04 | No clear response, unilateral referral |
| 05 | Incomplete - Screening contraindicated |
| 06 | Incomplete - Baby deceased |
| 07 | Incomplete - Baby unsettled |
| 08 | Incomplete - Parent declined screening |
| 09 | Incomplete - Appointment missed |
| 10 | Incomplete - Lack of service capacity |
| 11 | Incomplete - Lost contact |
| 12 | Incomplete - Out of screening coverage |
| 13 | Incomplete - Late entry |
| 14 | Incomplete - Equipment malfunction |
| 15 | Incomplete - Equipment not available |
| 16 | Incomplete - Parent withdrew consent |
Change to Attribute: Changed Description
This records the outcome of an Out-Patient Attendance Consultant.
National Codes:
| 1 | Discharged from CONSULTANT's care (last attendance) |
| 2 | Another APPOINTMENT given |
| 3 | APPOINTMENT to be made at a later date |
Change to Attribute: Changed Description
The smoking status of the PERSON at the time the TOBACCO USAGE is recorded.The SMOKING STATUS of the PERSON at the time the TOBACCO USAGE is recorded.
National Codes:
| 1 | Current smoker |
| 2 | Ex-smoker |
| 3 | Non-smoker - history unknown |
| 4 | Never smoked |
| 9 | Unknown |
References:National Cancer Data Set Version 1.1_ISB October 2001Acute Myocardial Infarction Core Dataset
Change to Data Element: Changed Description
| Format/length: | an1 |
| HES item: | |
| National Codes: | See BASAL CELL CLINICAL MORPHOLOGY |
| Default Codes: |
BASAL CELL CLINICAL MORPHOLOGY is the same as attribute BASAL CELL CLINICAL MORPHOLOGY.
Change to Data Element: Changed Description
| Format/length: | n1 |
| HES item: | |
| National Codes: | See BASIS OF DIAGNOSIS |
| Default Codes: | 9 - Not known |
BASIS OF DIAGNOSIS (CANCER) is the same as attribute BASIS OF DIAGNOSIS.
Change to Data Element: Changed Description
| Format/length: | n1 |
| HES item: | |
| National Codes: | See BLEED COMPLICATION CODE |
| Default Codes: | 9 - Unknown |
Notes:This is the same as attribute BLEED COMPLICATION CODE.BLEED COMPLICATION is the same as attribute BLEED COMPLICATION CODE.
Options in order of preference: use highest item that applies.
CCAD item name:
Bleeding complications
Change to Data Element: Changed Description
| Format/length: | an1 |
| HES item: | |
| National Codes: | See BONE SARCOMA LOCATION |
| Default Codes: |
BONE SARCOMA LOCATION is the same as attribute BONE SARCOMA LOCATION.
Change to Data Element: Changed Description
| Format/length: | n2 |
| HES item: | |
| National Codes: | |
| Default Codes: |
Notes:
The total number of Fractions or hyperfraction of a Brachytherapy Treatment Course .
Change to Data Element: Changed Description
| Format/length: | an1 |
| HES item: | |
| National Codes: | See BRACHYTHERAPY DELIVERY TYPE |
| Default Codes: |
BRACHYTHERAPY DELIVERY TYPE is the same as attribute BRACHYTHERAPY DELIVERY TYPE.
Change to Data Element: Changed Description
| Format/length: | n1 |
| HES item: | |
| National Codes: | See BRACHYTHERAPY DOSE RATE |
| Default Codes: |
BRACHYTHERAPY DOSE RATE is the same as attribute BRACHYTHERAPY DOSE RATE.
Change to Data Element: Changed Description
| Format/length: | an2 |
| HES item: | |
| National Codes: | See BRACHYTHERAPY ISOTOPE TYPE or UNSEALED SOURCE ISOTOPE TYPE |
| Default Codes: |
BRACHYTHERAPY ISOTOPE TYPE is the same as attribute BRACHYTHERAPY ISOTOPE TYPE for sealed sources or UNSEALED SOURCE ISOTOPE TYPE for unsealed sources.
BRACHYTHERAPY ISOTOPE TYPE is the same as attribute BRACHYTHERAPY ISOTOPE TYPE for sealed sources or UNSEALED SOURCE ISOTOPE TYPE for unsealed sources.
Change to Data Element: Changed Description
| Format/length: | n2 |
| HES item: | |
| National Codes: | |
| Default Codes: |
BRACHYTHERAPY PRESCRIBED FRACTIONS is the same as attribute BRACHYTHERAPY PRESCRIBED FRACTION.
Change to Data Element: Changed Description
| Format/length: | an2 |
| HES item: | |
| National Codes: | See BRACHYTHERAPY TYPE |
| Default Codes: |
BRACHYTHERAPY TYPE is the same as attribute BRACHYTHERAPY TYPE.
Change to Data Element: Changed Description
| Format/length: | an1 |
| HES item: | |
| National Codes: | See BREAST CANCER NURSE SEEN |
| Default Codes: | 9 - Not known |
BREAST CANCER NURSE SEEN is the same as attribute BREAST CANCER NURSE SEEN.
Change to Data Element: Changed Description
| Format/length: | n1 |
| HES item: | |
| National Codes: | See BROAD PATIENT GROUP CODE |
| Default Codes: |
BROAD PATIENT GROUP CODE is the same as attribute BROAD PATIENT GROUP CODE.
Change to Data Element: Changed Description
| Format/length: | n1 |
| HES item: | DISMETH |
| National Codes: | See DISCHARGE METHOD |
| Default Codes: | 8 - Not applicable - Hospital Provider Spell. not yet finished (i.e. not discharged) |
| 9 - Not known: a validation error |
Notes:DISCHARGE METHOD (HOSPITAL PROVIDER SPELL) is the same as the attribute DISCHARGE METHOD and the values recorded are the National Codes contained within the attribute definition with the addition of the Default Codes.
This records the method of discharge from a Hospital Provider Spell.DISCHARGE METHOD (HOSPITAL PROVIDER SPELL) is the same as the attribute DISCHARGE METHOD.
Hospital Provider Spell is an ACTIVITY GROUP where ACTIVITY GROUP TYPE is National Code 21 'Hospital Provider Spell'.
Change to Data Element: Changed Description
| Format/length: | n1 |
| HES item: | |
| National Codes: | See FIRST ATTENDANCE |
| Default Codes: |
Notes:
This indicates whether a PATIENT is making a FIRST ATTENDANCE or follow-up attendance or contact and whether the CONSULTATION MEDIUM USED was a face to face contact or telephone/telemedicine consultation.
A FIRST ATTENDANCE is the first in a series, or only attendance of an APPOINTMENT which took place regardless of how many previous APPOINTMENTS were made which did not take place for whatever reason. All subsequent attendances in the series which take place should be recorded as follow-up.
FIRST ATTENDANCE National Code 5 - "Referral to Treatment Clock Stop Administrative Event" allows the Secondary Uses Service to build accurate PATIENT PATHWAYS for the reporting of 18 weeks activity. It flows through the CDS V6 TYPE 020 - OUTPATIENT CDS structure. See Referral To Treatment Clock Stop Administrative Event.
Change to Data Element: Changed Description
| Format/length: | n1 |
| HES item: | |
| National Codes: | See FIRST REGULAR DAY OR NIGHT ADMISSION |
| Default Codes: |
FIRST REGULAR DAY OR NIGHT ADMISSION is the same as attribute FIRST REGULAR DAY OR NIGHT ADMISSION.
Change to Data Element: Changed Description
| Format/length: | n2 |
| HES item: | |
| National codes | See FLEXIBLE WORK PATTERN TYPE CODE |
| Default codes |
Notes:This is the same as attribute FLEXIBLE WORK PATTERN TYPE CODE.FLEXIBLE WORKING PATTERN TYPE CODE is the same as attribute FLEXIBLE WORK PATTERN TYPE CODE.
A classification of flexible working schemes for EMPLOYEES.
For enquiries, please email datastandards@nhs.net